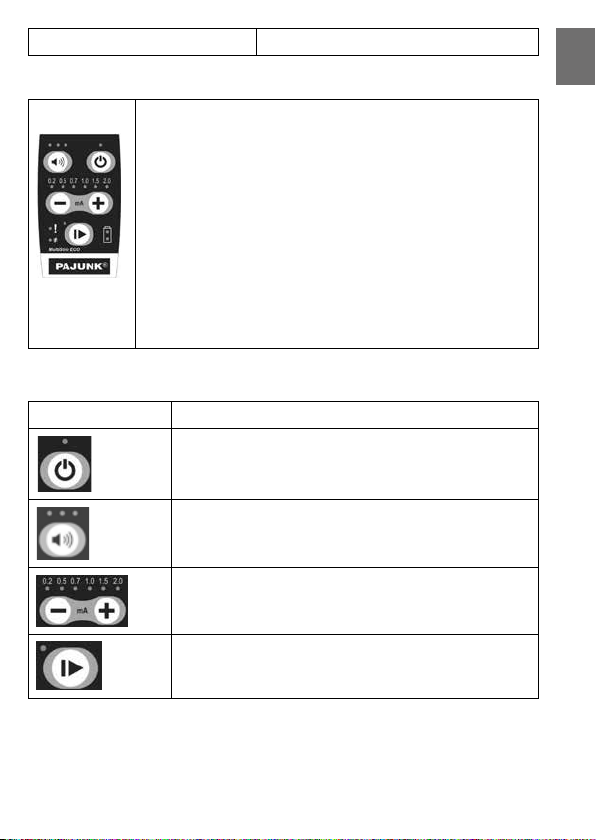
4
English
Complication
Typical complications of peripheral nerve blocks include:
• Toxic reactions (to injection solution)
• Late neurological damage
Specific complications related to certain block anaesthesia techniques may also
occur. Users should consider them based on the state of the art.
Warnings and safety instructions
Connect the connection socket of the stimulation cannula only to the mating
connector of the nerve stimulator. If you use an extension cable, please make
sure that it is correctly connected!
The snap on the back of the device may only be connected to the adhesive elec-
trode. By no means may these plugs/connections get in contact with live parts
(e.g. sockets) or metal objects.
Stimulation must not take place on the head, eyes, mouth or heart.
To avoid a gas explosion of anaesthetic gases or inflammation of flammable
liquids, the MultiStimECO must not be used in explosive atmospheres.
To avoid unintended injury to the patient, all connected equipment in the
patient's environment must comply with the applicable regulations. All equip-
ment and accessories must comply with requirements to EN 60601-1 and
EN60601-1-1 as well as the applicable sub-standards.
Please note that, in the worst case, all leakage currents and/or the patient's aux-
iliary currents might add up so that inadmissibly high values put the patient
at risk, even if all requirements for the individual devices are met. Therefore, it
must be checked in advance whether admissible limit values are exceeded when
equipment is interconnected. Improper interconnection of devices and equip-
ment (formation of systems) can cause life-threatening injuries to the patient.
The patient must not get in contact with metal objects which are earthed or
have an electrically conductive connection with other devices or enable capaci-
tive coupling. We therefore recommend using a suciently insulating, antistatic
cover for the operating table.
Under no circumstances may the MultiStimECO be used with other instru-
ments and accessories than those approved, supplied or recommended by the
manufacturer. Only PAJUNK® accessories have been tested for EMC (electro-
magnetic compatibility). Third-party accessories may lead to serious impair-
ment of the device and system features and cause permanent damage to
patient, user or device.
Do not make any unauthorised changes to the technical equipment of the
device. In case of manipulation, warranty and producer liability expire, and
patient safety is put at risk.