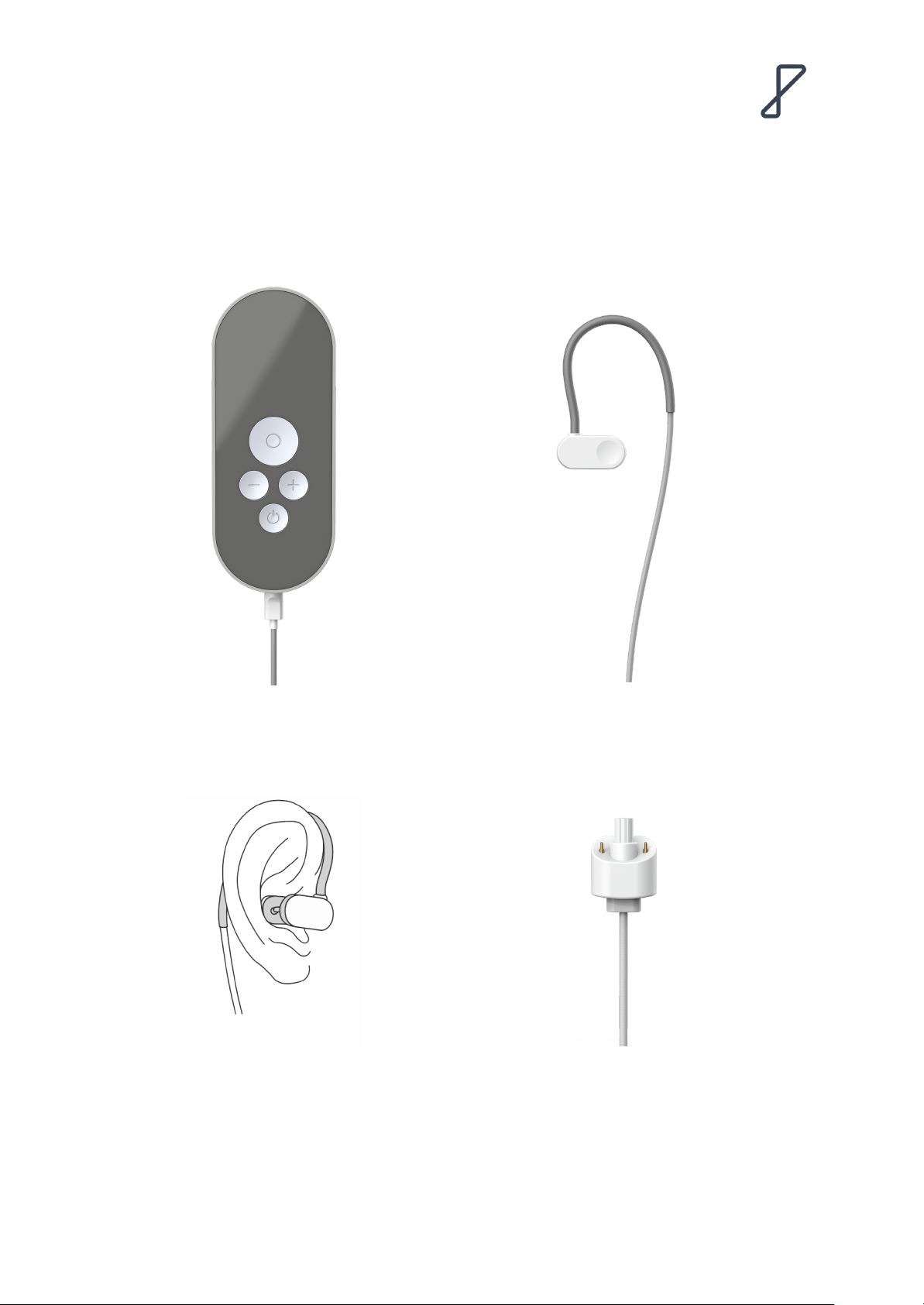
Precautions
Before Use:
- You must read the Nurosym Instructions for Use before using Nurosym. However, reading the
Instructions for Use may not be enough to fully explain the safe and effective use of the
device. Ask your HCP or contact Nurosym support if you have any questions about how to
use the device or require any further clarification of these Instructions.
- Only use Nurosym as described in these Instructions for Use or as otherwise directed by your
HCP.
- Remove jewellry that may interfere with the electrode location (earrings etc.) before using
Nurosym.
- Always carefully examine the device for any signs of damage or defects before use.
- Do not strip the batteries’ outer seal when inserting (for risk of short-circuiting), this can be
avoided by taking care when inserting.
- Do not share your Nurosym with another person.
Do not use Nurosym if:
- The skin on the tragus is broken or cracked.
- The device casing is cracked, dented, or appears to be damaged.
Discontinue use if you experience:
- Lightheadedness, dizziness, or chest pain.
- Excessive skin irritation.
Caring for Your Device:
- Do not pull leadwires to remove the electrode.
- Keep Nurosym away from water or other liquids.
- Keep Nurosym away from steam as moisture may damage the device.
- Store Nurosym in a safe location out of reach of children.
- Do not place the unit close to excessive heat.
- Do not open or take apart the case, or attempt to repair or modify the device. There are no
user serviceable parts. If the device is not working, contact Nurosym support.
- Keep the unit away from sources of high magnetic fields such as TV’S, microwave ovens and
hi-fi speakers, as these may affect the LED screen.
If the device seems to malfunction, when possible, contact Nurosym support for assistance. Nurosym
support cannot provide medical assistance.
Side Effects Associated with Nurosym
Nurosym has the highest safety profile of VNS technology, with 0 serious adverse events reported in
clinical study. Side effects are rare and typically resolve immediately after the stimulation is
complete.The most common side effects include:
Application site discomfort
Application site irritation/redness
Tingling on the skin where the device is applied (paresthesia/dysesthesia)
Potential Risks and Complications associated with vagus nerve stimulation
The following risks and complications have been associated with other devices delivering vagus nerve
stimulation including surgically implanted devices, and may potentially occur with Nurosym. In clinical
Privileged and confidential | Parasym Ltd | 5