SUMMARY
1KIT CONTENTS........................................................................................................................................ 3
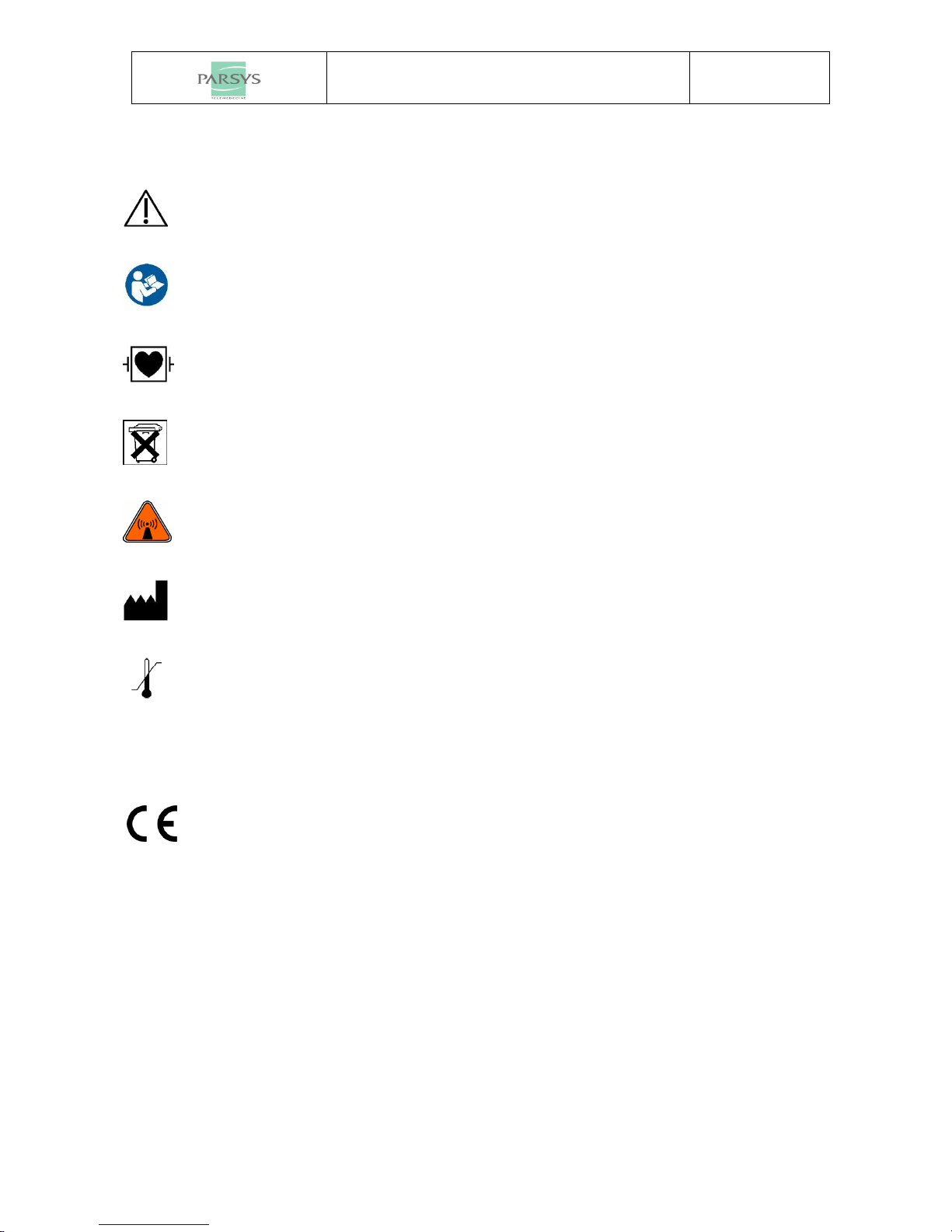
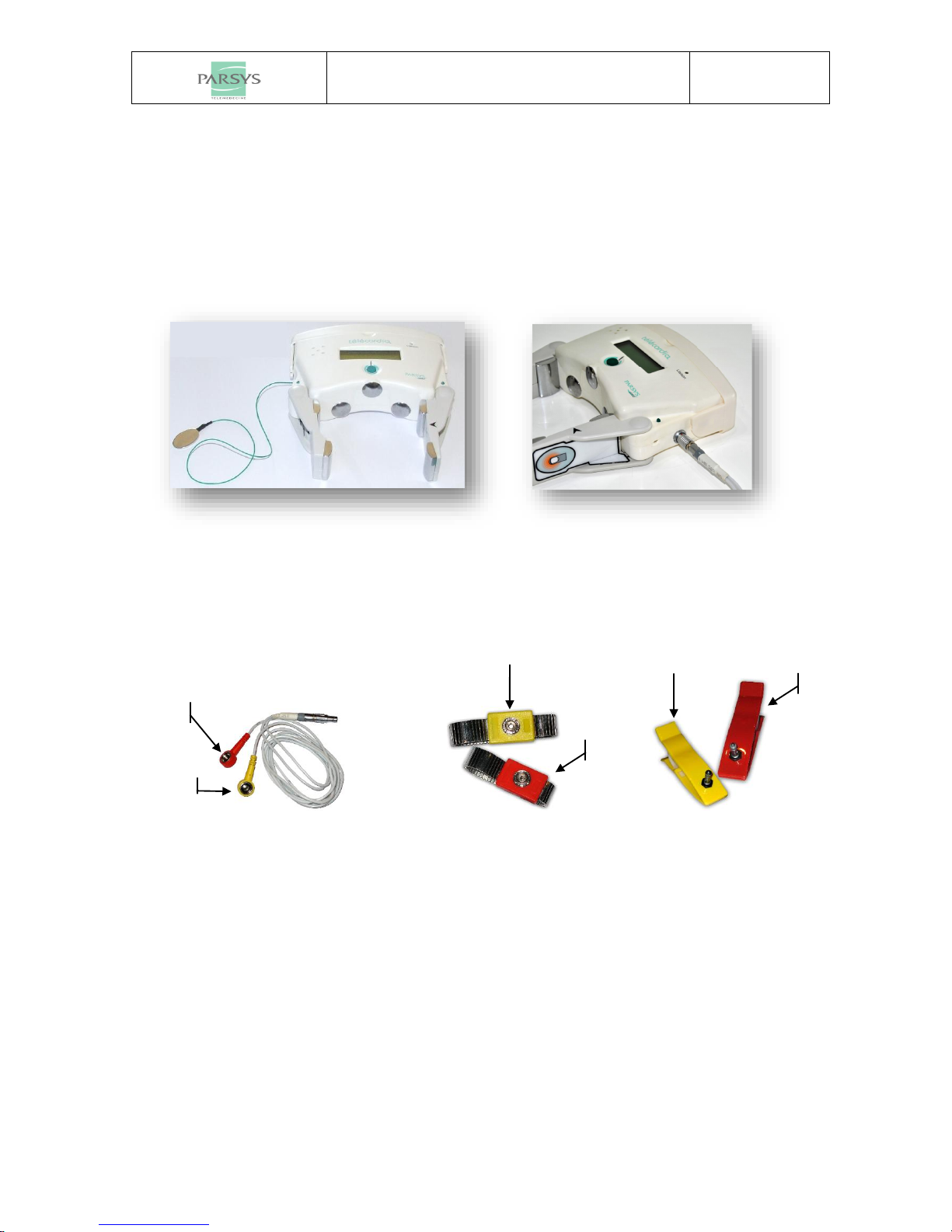
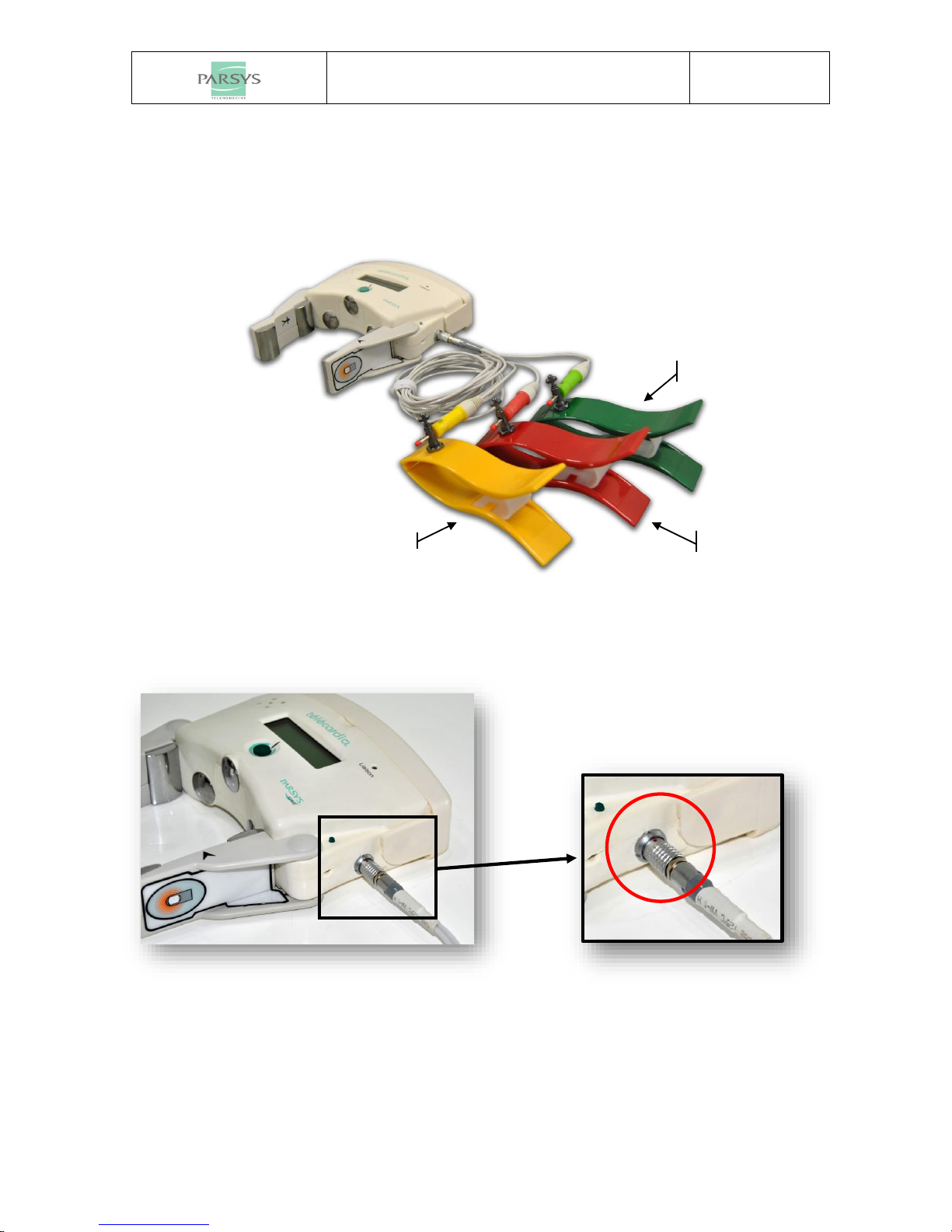
1.1 COMPONENTS......................................................................................................................................... 3
1.2 LIST OF SYMBOLS USED........................................................................................................................... 4
1.3 WARNINGS ............................................................................................................................................. 5
2EQUIPMENT DESCRIPTION................................................................................................................... 6
2.1 OVERVIEW.............................................................................................................................................. 6
2.2 ELECTRODES POSITION ........................................................................................................................... 6
2.2.1 Precordial leads ........................................................................................................................... 6
2.2.2 Peripheral leads........................................................................................................................... 7
2.3 DEVICE CONTROL BUTTONS..................................................................................................................... 9
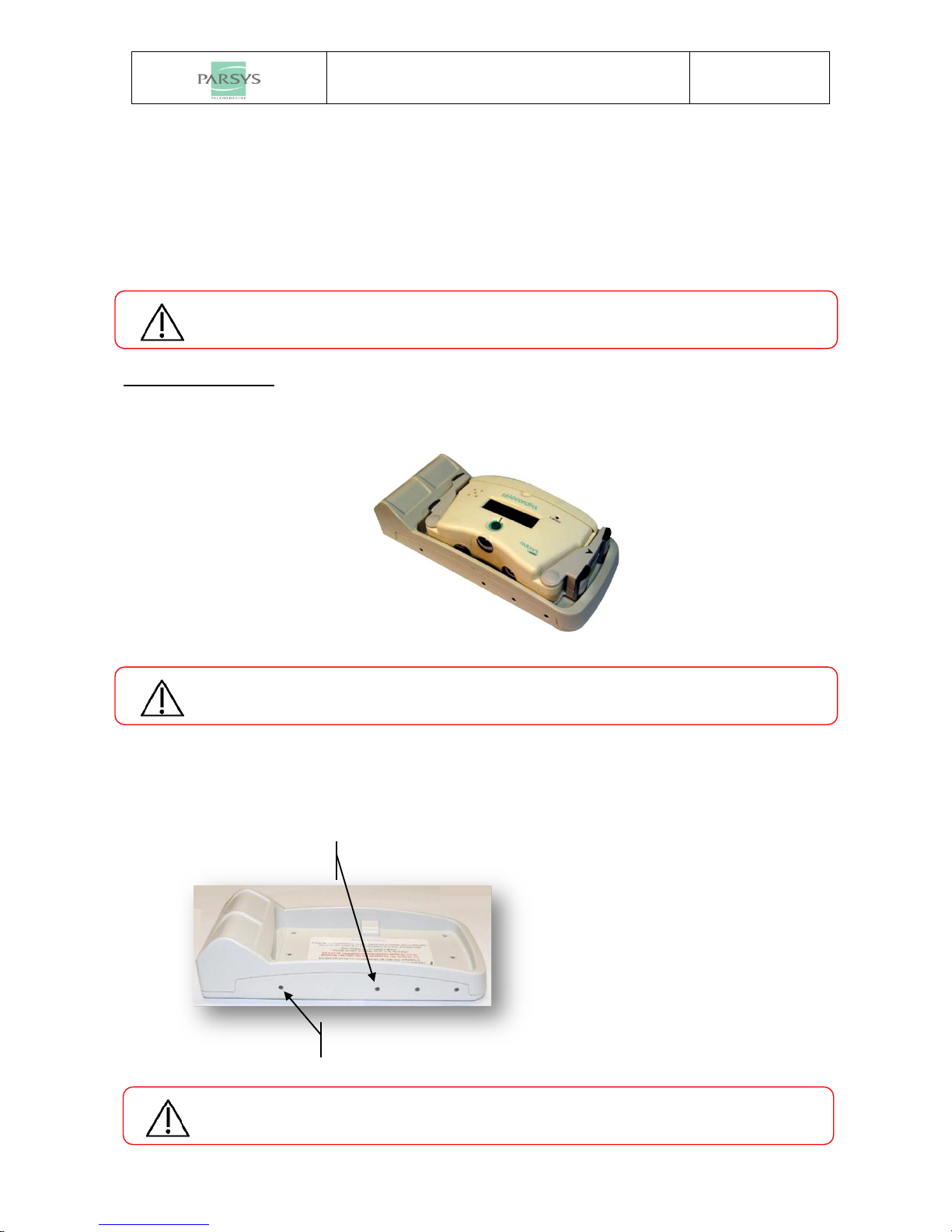
2.4 CHARGING BASE ................................................................................................................................... 10
2.5 ECG READING...................................................................................................................................... 11
3INSTALLATION OF TELECARDIALYSPC SOFTWARE..................................................................... 12
4BLUETOOTH PAIRING PROCEDURE OF THE TELECARDIA TO A PC........................................... 13
4.1 WINDOWS XP OPERATING SYSTEM................................................................................................... 14
4.2 WINDOWS 7OPERATING SYSTEM...................................................................................................... 25
4.2.1 Requirements............................................................................................................................. 25
4.2.2 Pairing of Telecardia with a Windows 7 PC............................................................................... 26
4.3 WINDOWS 8/8.1 OPERATING SYSTEM.............................................................................................. 37
4.3.1 Requirements........................................................................................................................... 37
4.3.2 Pairing of Telecardia with a Windows 8 / 8.1 PC .................................................................. 38
5USING TELECARDIALYSPC SOFTWARE.......................................................................................... 47
6ECG CAPTURE...................................................................................................................................... 48
6.1 2-LEAD PERIPHERAL PATIENT CABLE MODEL........................................................................................... 48
6.2 3-LEAD PERIPHERAL PATIENT CABLE MODEL........................................................................................... 54
7UPDATING THE PC SOFTWARE / DEVICE FIRMWARE.................................................................... 60
8TECHNICAL FEATURES....................................................................................................................... 61
8.1 GENERAL FEATURES ............................................................................................................................. 61
8.2 MECHANICAL FEATURES........................................................................................................................ 61
8.3 ELECTRICAL SPECIFICATIONS................................................................................................................. 61
8.4 FUNCTIONAL FEATURES......................................................................................................................... 62
8.5 FUNCTIONAL FLOW CHART..................................................................................................................... 63
9ELECTROMAGNETIC EMISSIONS....................................................................................................... 64
10 MAINTENANCE...................................................................................................................................... 67
10.1 CLEANING /DISINFECTION................................................................................................................. 67
10.2 PROCEDURE IN THE EVENT THAT THE INSTRUMENT IS DROPPED OR FALLS............................................ 67
10.3 RESET PROCEDURE.......................................................................................................................... 67
10.4 METROLOGICAL CHECKS................................................................................................................... 68
10.5 DISCHARGED BATTERY...................................................................................................................... 68
10.6 PRODUCT SCRAP TREATMENT ........................................................................................................... 68
11 USERS’ TRAINING ................................................................................................................................ 69
12 FAULT OR FAILURE ............................................................................................................................. 70
12.1 THE TELECARDIA ECG DOES NOT SEND ANY TRACE........................................................................... 70
12.2 THE ECG IS SUBJECT TO INTERFERENCE........................................................................................... 70
12.3 THE TELECARDIA ECG IS NOT CHARGING .......................................................................................... 70
12.4 THE TELECARDIA ECG REMAINS BLOCKED......................................................................................... 71
12.5 THE TELECARDIA ECG HAS LOST IS TIME STAMPING........................................................................... 71
13 PARSYS TELEMEDICINE WARRANTY AND AFTER-SALES SERVICE ........................................... 72