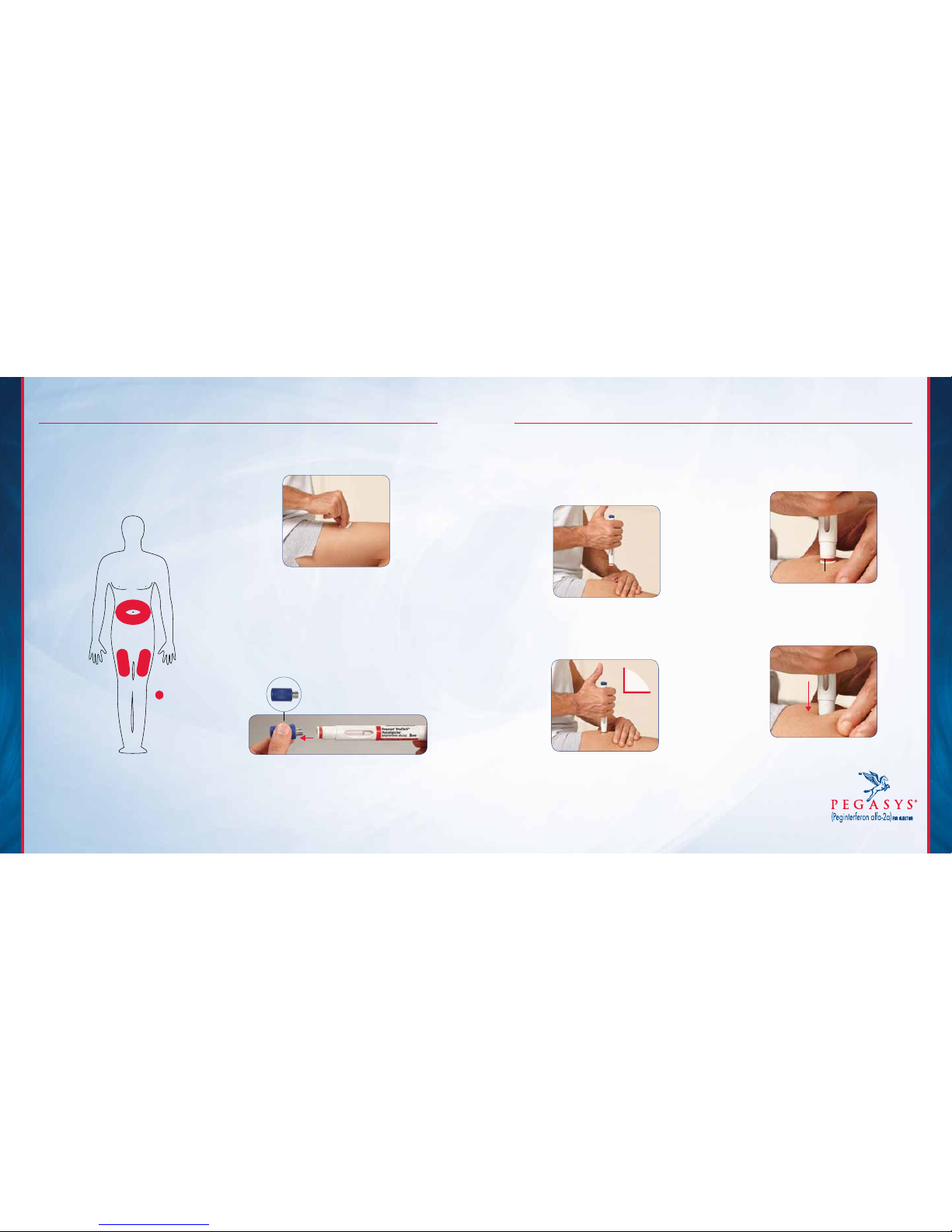
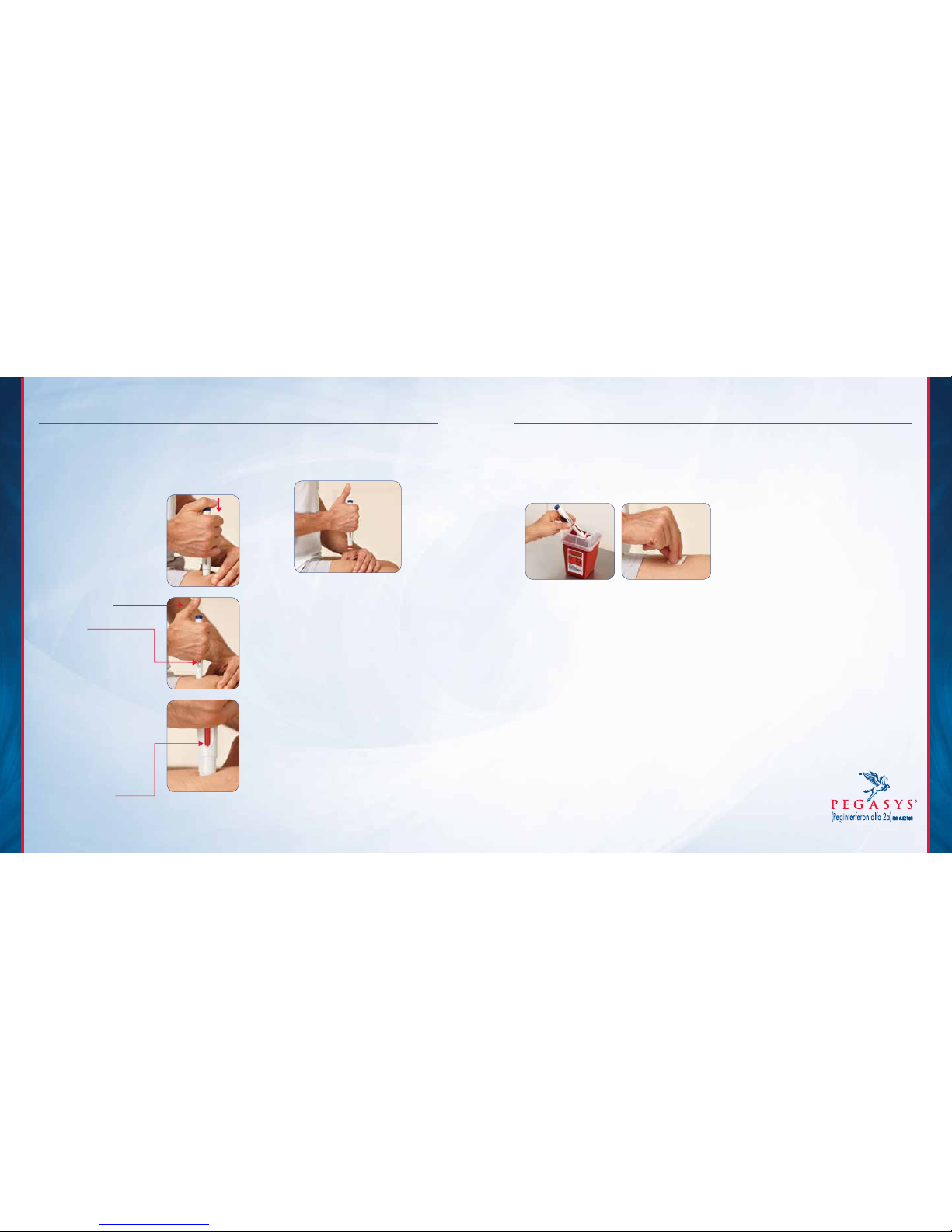
IMPORTANT SAFETY INFORMATION
PEGASYS, like other alpha interferons, may cause or
worsen fatal or life-threatening problems (like mental,
immune system, heart, liver, lung, intestinal and
infections). Your doctor should monitor you during
regular visits. If you show signs or symptoms of these
conditions, your doctor may stop your medication. In
many patients, but not all, these conditions get better
after they stop taking PEGASYS (see the Medication
Guide for more information and Warnings).
Ribavirin, including COPEGUS®, can cause birth defects
and/or death to an unborn baby. Female patients and
the female partners of male patients should avoid
getting pregnant. Ribavirin is known to cause anemia
(low red blood cells), which can make heart disease
worse (see the COPEGUS Medication Guide for more
information and Warnings).
What is the most important information I should
know about PEGASYS?
• COPEGUS in combination with PEGASYS may
cause birth defects or death of your unborn baby.
• PEGASYS therapy may cause you to develop mood
or behavioral problems.
• Some people who take PEGASYS alone or in
combination with COPEGUS may get heart problems.
• Stroke or symptoms of a stroke.
• Some people taking PEGASYS develop new or
worsening autoimmune problems.
• Some people who take PEGASYS may get an infection.
PEGASYS alone or in combination with COPEGUS can
cause serious side effects. Some of these side effects may
cause death. Tell your healthcare provider right away if
you have any of these symptoms while taking PEGASYS.
Do not take PEGASYS if you:
• have certain other liver problems
• have certain types of hepatitis caused by your immune
system attacking your liver (autoimmune hepatitis)
• have had a serious allergic reaction to another alpha
interferon medicine or to any of the ingredients in PEGASYS.
Do not take PEGASYS in combination with
COPEGUS if you:
• are pregnant, or planning to get pregnant during
treatment or during the 6 months after treatment
• are a male patient with a female sexual partner who
is pregnant or plans to become pregnant at any time
while you are being treated with COPEGUS or during
the 6 months after your treatment has ended
• have certain blood disorders such as thalassemia
major or sickle-cell anemia
• take didanosine (Videx® or Videx® EC)
Do not give PEGASYS to a baby under 1 year of age.
PEGASYS contains benzyl alcohol.
Before taking PEGASYS, tell your healthcare provider
about all your medical conditions or problems, and if
you are pregnant or breastfeeding, or plan to become
pregnant or breast feed during treatment with PEGASYS.
Tell your healthcare provider about all the
prescription and nonprescription medicines,
vitamins and herbal supplements you take.
PEGASYS can cause serious side effects including:
blood problems, thyroid problems, blood sugar
problems, serious eye problems, serious liver
problems, worsening of liver problems including
liver failure and death, lung problems, inammation
of your pancreas, inammation of your intestines,
serious allergic reactions and skin reactions, effect
on growth in children, and nerve problems.
The most common, but less serious, side effects of
PEGASYS include: u-like symptoms, tiredness
and weakness, stomach problems, loss of appetite,
skin reactions, hair thinning, trouble sleeping.
Tell your healthcare provider if you have any side
effect that bothers you or that does not go away.
Please see accompanying PEGASYS full
Prescribing Information and Medication Guide,
including Boxed WARNINGS, for additional
Important Safety Information.
PEGASYS® and COPEGUS® are registered trademarks of Hoffmann-La Roche Inc. All other brands for listed products are trademarks or registered
trademarks (as indicated) of their respective owners and are not trademarks of Genentech, Inc. or Hoffmann-La Roche Inc.
© 2011 Genentech USA, Inc. All rights reserved. PEG0000684901 Printed in USA. (11/11)