PEMF Systems 9K-N915 User manual

9K-N915
User Manual

Page 2
DO NOT USE:
l
If you are, or may be pregnant,
l
If you are receiving chemotherapy,
l
If you have cancer or malignant tumors,
l
If you have had surgery in the past 24 hours,
l
If you have hemorrhagic tendencies, Purpura or Hemophilia,
l
If you have major metabolic diseases uncontrolled by medication (HIV, ulcers, seizures…),
l
Within25cmofpacemakers,debrillatorsoranyotherimplantedelectronicdevices,
l
Within 25cm of metallic implants (stents, pins, rods, or screws),
l
Soonaftertakinganymedicationastheireffectsmaybeintensied.
Consult your health care provider to discuss using the device if you think you are at risk for any of
thesecontraindications.
CONTRAINDICATIONS
INDICATIONS FOR USE
Thisdevicehasatreatmentcoilconnectedtoacontrolunitwhichgeneratespulsedelectromagneticelds
(PEMF).ThetreatmentcoiltransfersthetherapeuticPEMFtothebodyinvedifferentintensitylevels.
Clinicalresearchhasdemonstratedthatthisdeviceissafeforusetotreatinamedandpainfulareasofthe
bodywhenusedaccordingtothe“DirectionsforUse”inthismanual.
DESCRIPTION
FREQUENCY OF USE
Thereare4differenttreatmentcycles:5min.,10min.,15min.and20minutes.
Ontherstuse,therecommendedtreatmentisone5min.cycleperareatreated.Thereafter,thetreatment
durationcanbeincreasedanddifferentareascanbetreatedduringatreatmentcycle.
Additional treatments of the same area on the same day are not hazardous but do not offer additional
therapeuticbenets.
Control unit
Medical grade MAINS power cord
20cmdiameterdoublelooptreatmentcoil
30cmdiametersinglelooptreatmentcoil
User Manual
Warranty Registration Card
WHAT’S INCLUDED IN THE BOX DEVICE SPECIFICATIONS
Voltageinput: 120VAC,60Hz
Current input: per device
Dimensions: per device
Weight: per device
Shipping weight: per device
Temporarilyreducespainandinammation,andtemporarilyimprovesrangeofmotionoftheareatreated.

Userswithheartconditionsshouldconsulttheirphysicianbeforeusingthedevice.
Users with suspected or diagnosed epilepsy should consult their physician before using
thedevice.
Donotplacethetreatmentcoiloverasuturelinewithin3daysaftersurgery.
Users taking pain, anxiety, depression or any other medication should be carefully monitored when
usingthedeviceasmedicationeffectivenessmaybeintensied.
Applyingdirectlyoverthemenstruatinguterusmaycauseincreasedbleeding.
Donotapplyathighintensityoverareasoftheskinorbodythatlacknormalsensation.
Userswithlowbloodpressuremayfeeltemporarilydizzywhenrststandingupaftertreatment.
Metal objects,
jewelry and metal chains
will heat with prolonged exposure to pulsed electromagnetic
elds.Usethedeviceforone5-minutetreatmentcyclefollowedbyaveminutepausewithin25cmof
anyimplantedmetalobjectsbeforetreatingagain.
DangerousVoltageinside,DONOTtamperwithoropenthecontrolunit.
DO NOT USE the device near credit cards, security access cards, car keys, hearing aids, watches,
cellphones,iPODS,laptops,remotecontrols,oranyotherelectronicmedia.Theelectromagnetic
eldsmaydisrupttheirfunctioningand/ordemagnetizethem.
DO NOT USE while operating any machinery or during any activity in which involuntary muscle
contractionsmayputtheuseratundueriskofinjury.
DONOTUSEinwetenvironments.Donotimmerseanypartoforpouranyliquidsonthedevice.
Keepawayfromsourcesofheatandmoisture.
Keep the device out of the reach of children! Children may be at risk of strangulation with the power
cordand/ortreatmentcoilpigtailsandriskasphyxiationwiththepackingmaterials.
PRECAUTIONS
ADVERSE REACTIONS
There are no known negative side effects, or reported adverse or allergic reactions with the use of this
device.
Detoxicationmayoccur,drinkplentyofwateraftertreatment.
Overusemaycausemusclesoreness.
In case of any adverse reaction, stop using the device andconsultaphysician.
Page 3
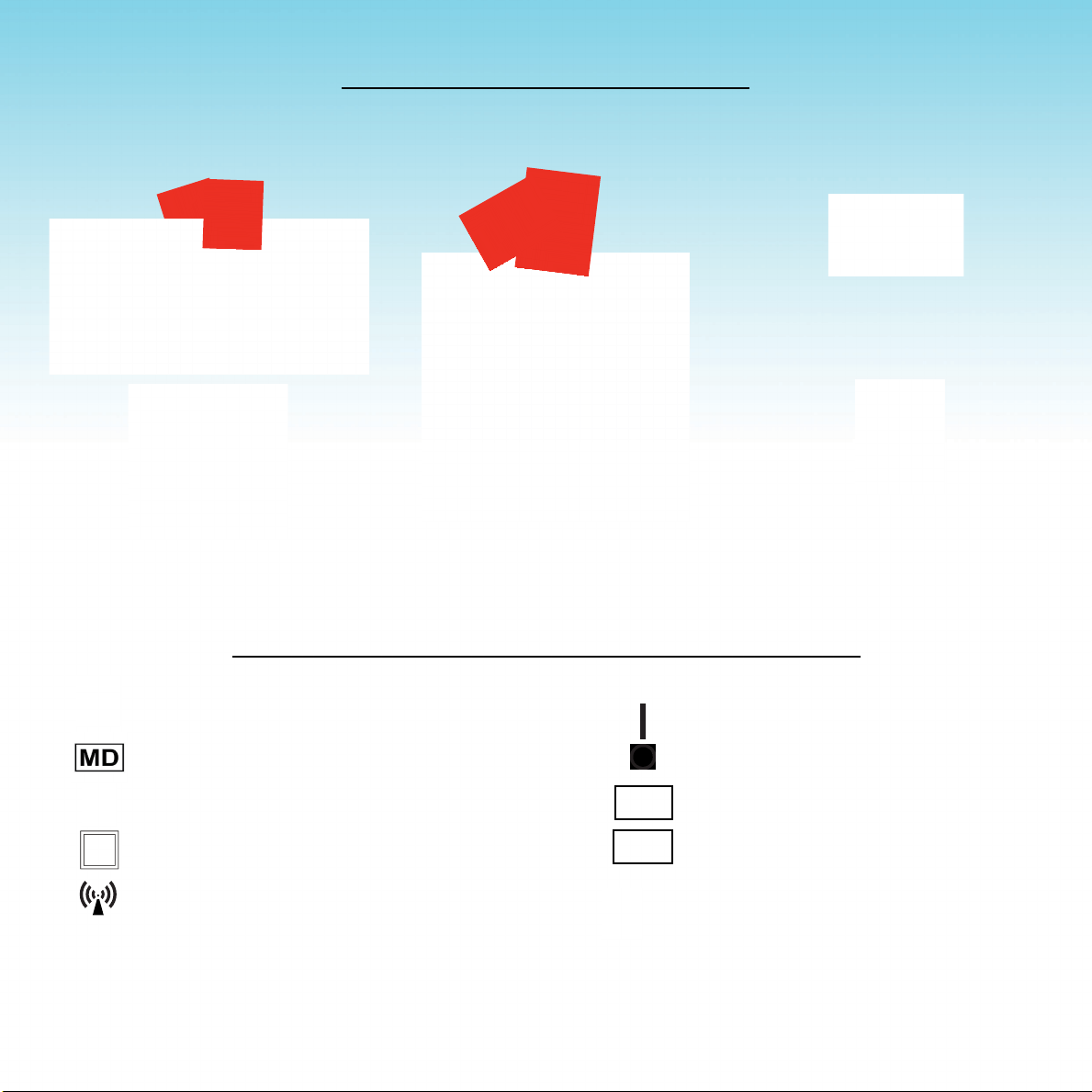
DEFINITION OF SYMBOLS, LABELS AND MARKINGS
20XX
SN
Read the entire User Manual BEFORE using
the device
Medical Device
Type B Applied Parts
TypeIIEquipment
Non-ionizingRadiation
REF
MAINS Power ON
MAINS Power OFF
Catalogue or Reference Number
Serial Number
Manufacturer’sIdentication
with Date of Manufacture
USER CONTROLS AND SYMBOLS
Pair of coil connector sockets allow one
treatment coil to be safely and securely
clockedintoplace.
Preset5,10,15or20minutespushbuttons;pressonetobeginatreatment.
Blue lights illuminate when a cycle begins and turn off incrementally during
treatment.Allbluelightsturnoffwithabeepattheendofthetreatment.
MAINS socket to plug
in the medical grade
powercord.
Page 4
The MAINS power
rocker switch turns
the device on “I” and
off“O”.
Intensity Dial: always set at lowest setting
beforebeginningtreatment.Increasetoa
comfortablelevelaftertreatmentbegins.
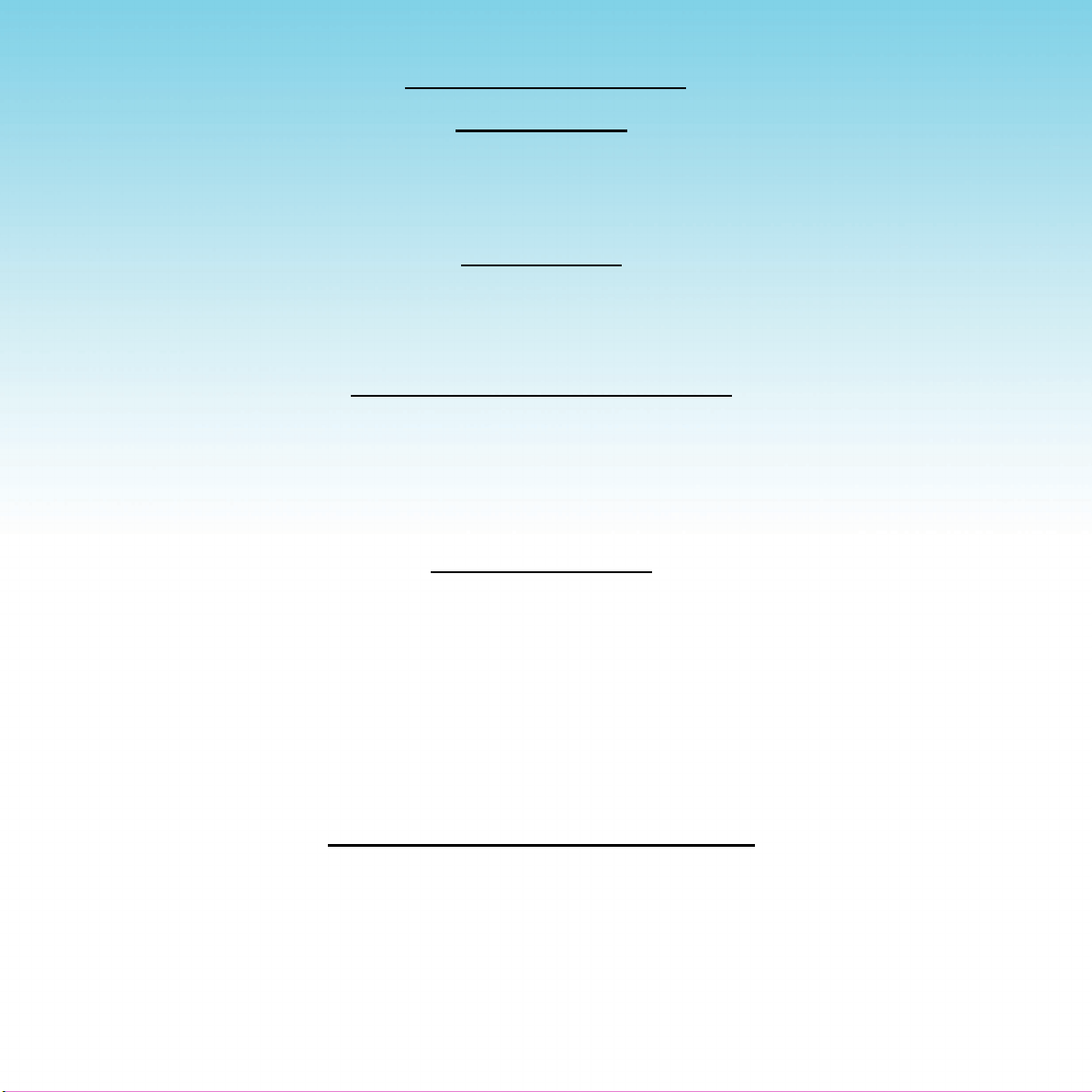
DIRECTIONS FOR USE
Treatment Cycle
A treatment cycle lasts 5,10,15or20minutes.Pressthedesiredpushbuttontobeginatreatment.The
treatmentintensitymaybesettoacomfortablelevelforeachareatreatedusingtheintensitydial.Setthe
intensity at its lowest level before beginning any treatment and increase the intensity until the desired level
isreachedaftertreatmentbegins.
Getting Started
Connect the power cord into the MAINS socket on the front of the
device
.
Always lock a treatment coil connector into the two connector sockets on the front of the device before turning
theMAINSpowerswitchon“I”.The
device
willnotstartifthetreatmentcoilisnotproperlyconnected.
Inserting/Changing a Treatment Coil
To connect a treatment coil, push one of its connectors with the silver tab up into one of the coil connector
socketsonthefrontofthedeviceandrotateitclockwiseuntilitclicks.Repeatwiththeotherconnectorinto
theothersocket.Thetreatmentcoilisthenlockedintoplace.Toremovethetreatmentcoil,slidethesilver
tabofeachconnectorbackandturntheconnectorcounter-clockwise,thenpullitout.
Donotplugorunplugthetreatmentcoilduringanactivetreatmentcycle.
Beginning Treatment
Press the MAINS power rocker switch to “I” to turn the
device
on.
Placeatreatmentcoilonoraroundthedesiredtreatmentarea.Thecloserthecoilistothetreatmentarea,
themoreeffectivethetreatmentwillbe.Userscanremainfullyclothedandnodirectcontactbetweenthecoil
andtheskinisnecessaryforthetreatmenttobeeffective.
Beforestartinganytreatmentonanyarea,settheintensityleveltothelowestlevel.Pressthe5,10,15or
20min.pushbuttontobeginatreatment.Increase.theintensitytoacomfortablelevelaftertreatmentbegins.
Bluelightswillturnonthenoffincrementally.Alllightsturnoffwithalongbeepattheendofthetreatment.
Press a 5,10,15or20min.pushbuttonagaintobeginanothertreatmentcycle.
Pausing/Resetting/Ending the Treatment
Duringthetreatmentcycle,brieypresstheactivetreatmentpushbuttontopausethetreatment.The
correspondingpushbuttonbluelightashescontinuouslywhilein“pausemode”.Toresumethetreatment
cycle,brieypressthesamepushbuttonagain.
To end the treatment cycle prior to completion, press and hold the active treatment pushbutton until you hear
asinglelongbeepandallthebluelightsturnoff.
Page 5
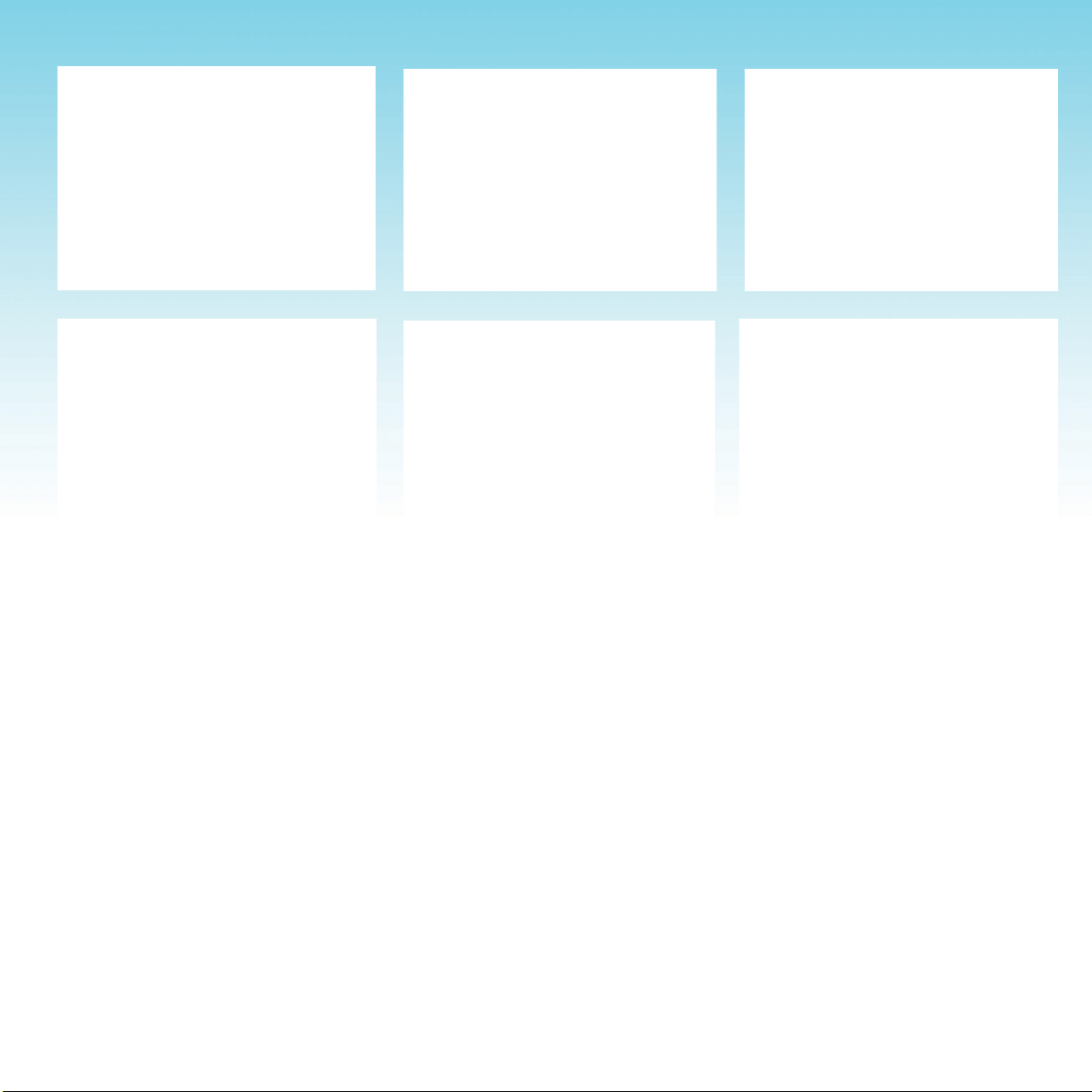
FOOT ANKLE ANKLE
KNEE KNEE KNEE
ELBOW ELBOW
HAND & WRIST NECK NECK
KNEE
Page6
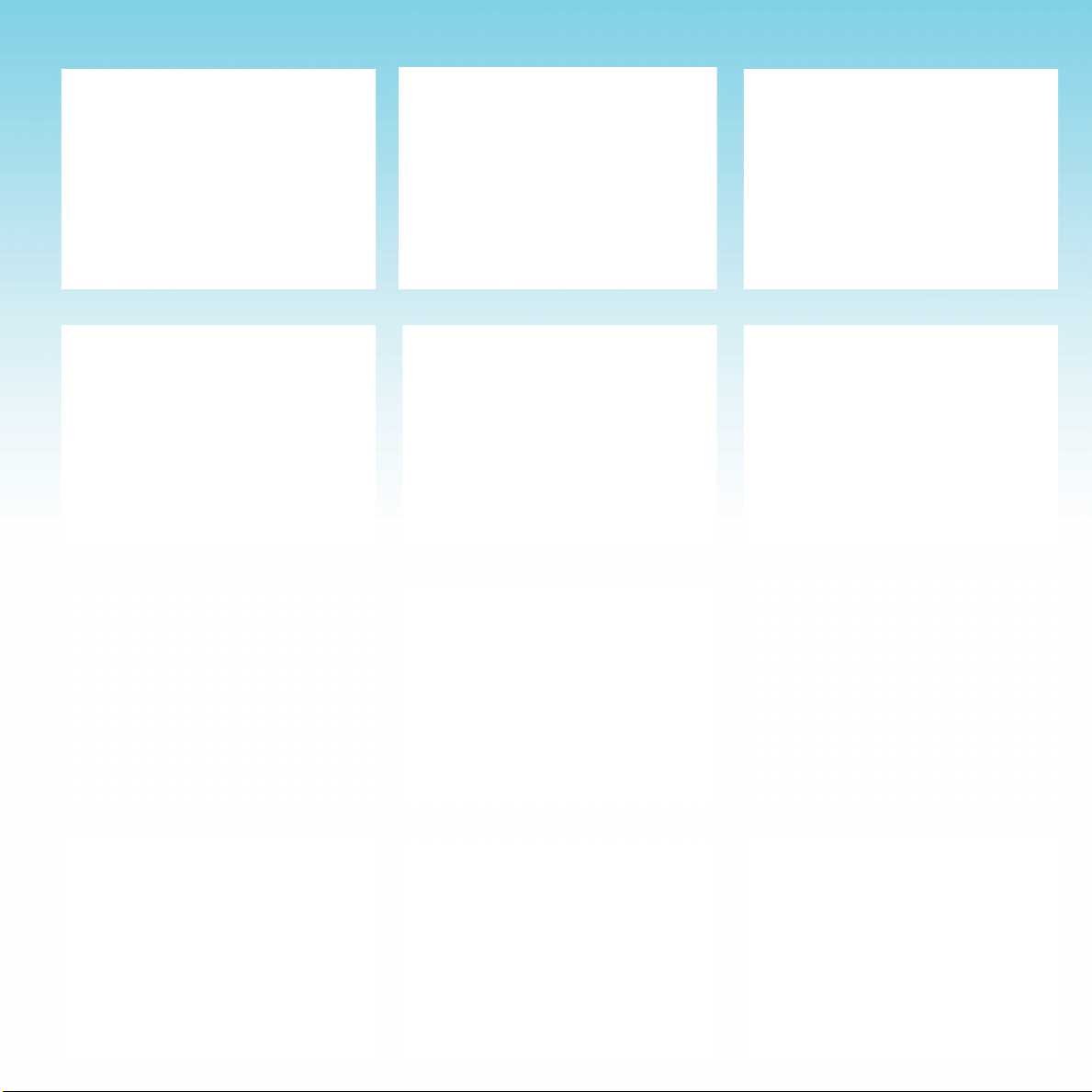
SHOULDER SHOULDER CHEST & SHOULDER
ABDOMEN CHEST UPPER BACK
MID BACK MID TO LOW BACK MID TO LOW BACK
LOW BACK HIPS HIPS
Page 7

Page 8
TROUBLESHOOTING
If the device fails to function after following the instructions detailed in the “Directions for Use” on page 4, use
the following troubleshooting steps:
If there are beeping sounds:
Proceedtoidentifythebeepsequencethedeviceemits:
l
Single two second beep =
End of session
Solution:Pressastartpushbuttontobeginanewtreatment.
l
Three short beeps & one long beep =
The coil is not connected properly or was disconnected
while the device was in use
Solution: Connect the coil properly following the instructions on page 4 and press a start
pushbuttontobeginatreatment.
l
Two short beeps three times followed by one long beep =
Device overheating
Solution:Allowthedevicetocooldownfor10minutesbyleavingthepoweronwhilenotreatment
isactivated.Thecoolingfanwillcoolthedevice.Pressastartpushbuttontobeginatreatment
afterthe10minuteshaveelapsed.
If the lights do not illuminate and there are no beeping sounds:
l
Make sure the MAINS power cord is properly connected at the control unit and a
MAINSoutlet.
l
MakesuretheMAINSoutletisfunctional.TrypluginganotherelectricalapplianceintotheMAINS
outlettodetermineifitworks.Iftheoutletworks,testthedevicewithanotherMAINSpowercord.
Ifthedevicestillfailstostartorfunctionproperly,contactthemanufacturer,listedonpage12togetaRe-
turnMerchandiseAuthorization(RMA)number.Disconnectthecoilandthepowercord.Packagethedevice
securelyandreturnittothemanufacturerforservicingwiththeRMAnumberandyourcontactinformation.

Keepthedevicedryandstoreinadryplace.Storageinadampplacemaycausecorrosion.
Store and transport at temperatures between 5oto50oCatarelativehumidityofupto93%non-condensing.
Remove the MAINS power cord and the detachable treatment coils from the control unit and pack all parts of the
device
securelybeforetransporting.
Service
The
device
hasnouserserviceablepartsandmustbereturnedtothemanufacturerforservicing.
Treatmentcoils,matsandMAINSpowercordsareavailablefromthemanufacturer.
Thedeviceisasealedunitandlint,dust,light,includingsunlight,etc.havenoeffectsonit.
DangerousVoltageinside;DONOTremovethecoverortamperwiththedevice.
REMOVINGTHECOVERORTAMPERINGWITHTHEDEVICEVOIDSTHEWARRANTY.
Contactthedistributorforassistanceinsettingup,usingormaintainingthedevice.
Thereisnomandatoryorscheduledcleaning,maintenanceorsterilizingnecessary.
If you choose to clean the device:
l
Disconnect the device from the MAINS before cleaning,
l
DONOTimmerseanypartorpouranyliquidsonthedevice.
Disposal
Cleaning
This device
isanelectronicdevice.Electronicsshouldneverbedisposedofwithregulartrash.Take
non-workingelectronicstoanelectronicsrecyclingcenter.
MAINTENANCE
STORAGE & TRANSPORTATION
Page 9

ELECTROMAGNETIC Immunity EMI
Page10
Table of contents
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual











