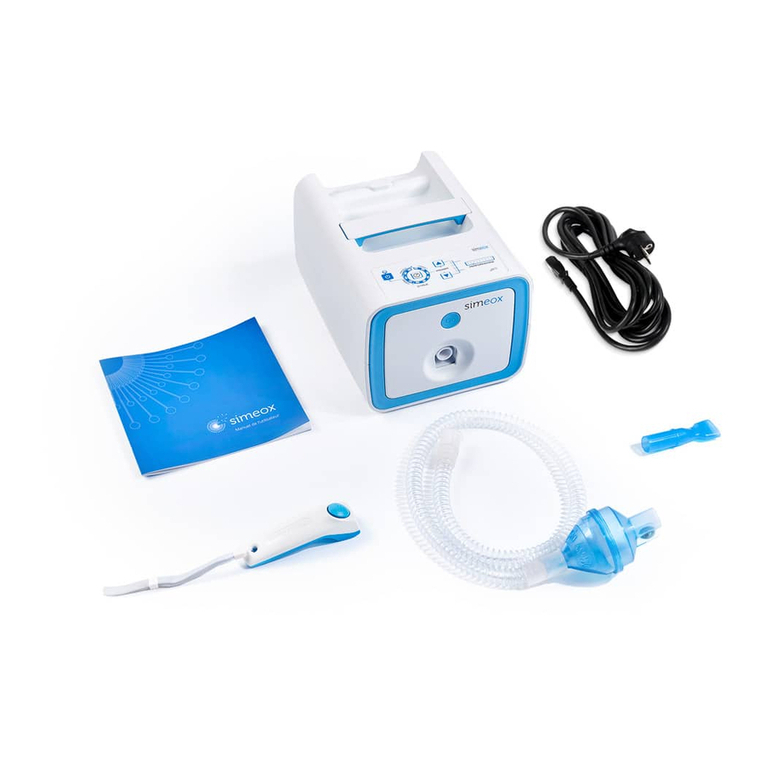
8
Before cleaning, always switch o the device and
unplug it from the wall socket.
When cleaning or disinfecting expiratory kits, make
sure to remove all product residues.
Some chemical products used as disinfectants can
harm the organism and the expiratory kits of the
device.
After cleaning and drying, store the device and
its accessories in its carrying bag, in a dry place,
protected from sunlight and dust, at room
temperature.Please refer to the section in this manual
on storage temperatures for SIMEOX.
The Lithium button battery in the remote control
presents an ingestion risk, to avoid this risk take the
following precautions:
- Do not store new batteries within the reach of
children
- Do not store used batteries in your home, take
them to a collection point
- Do not replace the batteries in the presence of a
child
- Do not let children play with the batteries
- Never put the battery in your mouth to test it or
to keep your hands free
- Never place the battery beside your medicines, to
avoid mistaking it for a tablet
Ingesting a button battery may cause the
following injuries:
- necrosis and perforation of the oesophagus
through mechanical compression
- electrical burns caused by the heat from the
electrical current between the mucosa and the
battery.
In the event that you ingest a button battery
contact a doctor or the emergency services
immediately.
The base of the SIMEOX housing incorporates
openings intended to aerate the device and to
evacuate any condensed moisture.
At home in particular, make sure that no objects,
especially metal objects such as staples, paperclips,
etc. are introduced into the housing through these
openings.
Although the live parts cannot be accessed through
these openings, a small object could remain stuck in
the device and cause a short-circuit.
The wrist strap supplied is intended to ensure that
the remote control is held securely at the wrist
during treatment sessions, preventing it from being
dropped.
It is not pre-installed.
The user is free to install it if he chooses, depending
on the estimated contamination risk.
Keep the power cord away from all hot surfaces: the
heat could degrade the protective sheath and cause
a failure, or a risk of electric shock.
Do not position SIMEOX in such a way that it is
dicult to use the disconnection device (the male
mains plug).