piomic COMS One User manual

Ref.: 01.0000
COMS One
Instructions for Use
DE Gebrauchsanweisung
FR Mode d‘emploi
IT Istruzioni per I‘uso
ES Instrucciones de uso

2
X. KAPITELNAMEASSISTANCE FOR LAY USERS / HILFE FÜR LAIENANWENDER
ASSISTANCE POUR UTILISATEURS PROFANES / ASSISTENZA PER UTENTI
COMUNI / AYUDA PARA USERIOS LEGOS
EN: Please read the entire instructions for use before trying to operate this device.
If you have any questions about your COMS One system, please contact your
healthcare professional at the number below:
DE: Bitte lesen Sie die Gebrauchsanweisung vollständig durch, bevor Sie dieses Gerät in
Betrieb nehmen. Wenn Sie Fragen zu Ihrem COMS One System haben, wenden Sie sich bitte
unter der untenstehenden Nummer an das medizinische Fachpersonal:
FR: Lire l’intégralité du mode d’emploi avant d’essayer d’utiliser ce dispositif médical. Pour
toute question au sujet de votre système COMS One, veuillez vous adresser à votre pro-
fessionnel de santé au numéro ci-dessous:
IT: Leggere l’intero manuale di istruzioni prima di provare a mettere
in funzione il dispositivo. Per qualsiasi domanda sul sistema per la COMS One, contattare il
proprio medico al seguente numero:
ES: Lea las instrucciones de uso en su totalidad antes de empezar a utilizar este dispositivo. Si
tiene alguna pregunta sobre el sistema COMS One, póngase
en contacto con su profesional sanitario en el siguiente número:
EN :Healthcare professional contact information / DE: Kontaktinformation medizinischen Fachpersonal
FR: oordonnées du professionnel de santé / IT: Dati di contatto del medico/
ES: Información de contacto del profesional sanitario

3
ENGLISH
1. INTRODUCTION
The COMS One Therapy System for Combined Optical and
Magnetic Stimulation, hereinafter called “COMS One”, is
approved exclusively for the use as specied in these
Instructions for Use.
Please read the information and note that these Instructions
for Use must be kept with the device.
The compact and portable COMS One offers simple and
comfortable wound therapy. Its use of optical and magnetic
stimulation is clinically proven to be effective in promoting
wound healing.
Intended purpose/Indications (When to use the device)
The COMS One Therapy System is intended to promote
wound healing by combined optical and magnetic
stimulation, in addition to standard of care. Indications for the
COMS One Therapy System are chronic leg and foot ulcers.
Contraindications (When not to use the device)
• Active skin cancer, history of skin cancer or any other
localized cancer, precancerous lesions or large moles in
the areas to be treated
• Pregnancy
Intended User Population
The COMS One should only be operated by properly in-
structed adults. Lay users including patients shall only use the
device upon instruction by a professional. All users shall not
be hard of hearing or deaf, must have adequate visual facul-
ty and shall be comfortable with the use of electronic devices.
Intended patient population
The COMS One is intended to be used on patients only
exhibiting conditions as described in the indications for use.
Intended Use Settings
The COMS One system is intended for use in professional
healthcare and home care settings.

4
X. KAPITELNAME2. WARNING AND SAFETY INSTRUCTION
WARNING
• Indicates a potentially hazardous situation which, if not
avoided, could result in death or serious injury.
CAUTION
• Indicates a potentially hazardous situation which, if not
avoided, could result in minor or moderate injury.
Safety related tip
• Indicating useful information about the safe use of the
device
The COMS One is intended for use as described in these
Instructions for Use.
Piomic only takes responsibility for the effect on safety,
reliability and performance of the COMS One if it is used in
accordance with the Instructions for Use.
Please read and observe these cautions and safety in-
structions before operation. Note that these Instructions for
Use are a general guide for the use of the product. Medical
situations must be addressed by a physician.
These Instructions for Use must be kept with the device.
WARNING
• Pacemaker operation may be adversely affected by
exposure to pulsed electromagnetic elds. Physicians
should not prescribe a therapy in case the treatment is
in close proximity to the pacemaker in order to prevent
malfunctioning of the pacemakers.
• Do not re-use articles labelled “single-use” in order to
prevent from infections and cross-contaminations.
• Do not use consumable when the sterile packaging is
damaged or has expired. Using unsterile consumables
can lead to infections.
• Unplug the device before cleaning and disinfection. It
may result in electric shock if the device is plugged in

5
ENGLISH
during cleaning and disinfection.
• Do not clean, disinfect or perform other service and
maintenance tasks during use of the device.
• Do not apply the device while it is charging. Non-
observance can lead to electric shocks.
• Do only use the original power supply certicated with
IEC 62368 -1 and USB cable for charging. Using other
power supplies or cables may result in electric shock or
electromagnetic interference that prevents the device or
other devices from operating properly.
• Store the system out of the reach of children and babies
to minimize the risk of strangulation from cables and
straps
• The strap is not sterile. Cover other wounds in proximity
of the strap before xating the strap in order to prevent
infections.
• Do not open or modify the device. Non-observance can
lead to electric shocks.
CAUTION
• Do not immerse the device directly in water or other
liquids (not suitable for use while bathing, showering)
and do not use in a hazardous explosive environment.
Non-observance can lead to electric shocks.
• Do not use accessories other than those specied or sold
by Piomic in order to ensure therapeutic performance.
• Fix the strap carefully to ensure no pressure oedemas are
created.
• Do not use any damaged parts in order to ensure thera-
peutic performance.
• Do not apply device more than once within 12 hours on
the same location to prevent overstimulation of the tissue.
• Do not apply device in case of known allergies to
incorporated surface materials (Silicone, Elastan,
PolyAmid, Polypropylen, Synthetic Rubber) to prevent
allergic reactions.
• Use of controls or adjustments or performance of pro-
cedures other than those specied herein may result in
hazardous radiation exposure.
• Do not use the device if you use photosensitizing agents
/ medications in order to prevent from photo-allergic

6
X. KAPITELNAME reactions.
• Do not look directly into the light source on the bottom of
the device. It may result in eye injuries.
• Do not operate HF (high-frequency) surgical equipment
in combination with the device. It can inuence the
operation of the device.
• Do not use the device if it is closer than 30 cm
(12 inches) to wireless communications equipment, such
as wireless home network routers, mobile phones, cord-
less telephones and their base stations. This can prevent
the device from working properly.
• Do not use the device adjacent to or stacked with other
equipment. If adjacent or stacked use is necessary, the
device should be observed to verify normal operation in
the conguration in which it will be used.
Safety related tip
• Do not dry the device with a microwave in order to
prevent from device damage.
• Do only use cleaning and disinfection agents as descri-
bed in the section “Cleaning and Disinfection” to prevent
device damage
• Do not use the device in proximity to an MRI (Magnetic
Resonance Imaging) scanner. Non-observance can lead
to considerable danger.
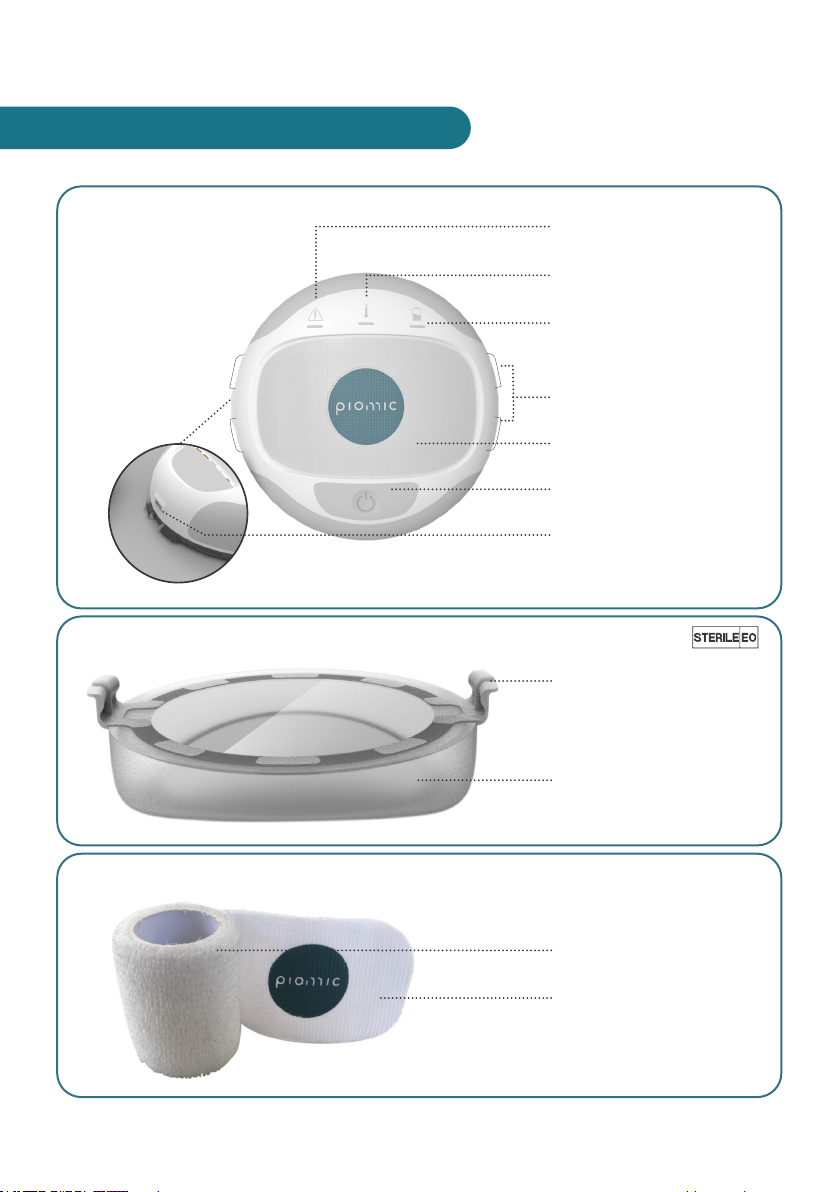
7
ENGLISH
Warning Indicator
Temperature Indicator
Battery Indicator
COMStouch Interface
COMSx/COMSrex
Interface
On/Off Button
Charging Socket
COMS One (Ref. 01.0000)
COMStouch (Single Use/Applied Part /Ref. 01.0001)
COMSx (Single Use/Ref. 01.0002)
Interface to COMS One
Device
Interface to patient
Strap (self adhesive)
Loops
3. MAIN ELEMENTS OF THE SYSTEM

8
X. KAPITELNAME
COMSrex (Single Patient Use/Ref. 01.0004)
Charger (Ref. S008ACM0500200)
Strap
Loops
Hooks
Upon delivery, check the COMS One system for complete-
ness and general condition.
The COMS One system was veried in combination with the
accessories listed above. For correct and safe operation use
the COMS One with Piomic accessories only.
COMS One Power Supply
USB Cable
Plugs

9
ENGLISH
4. BATTERY CHARGING
The charger has not been IP tested. Please charge device at a
dry location.
The USB cable can only be used for charging COMS One
with the charger and COMS One shall not be connected to
computers or other devices with the USB-cable.
1. Select plug matching your wall outlet and attach it to the
charger (initial use only).
CAUTION
• Do not apply the device while it is charging. Non-
observance can lead to electric shocks.
• Do only use the original power supply certicated
with IEC 62368-1 and USB cable for charging.
Using other power supplies or cables may result in
electric shock or electromagnetic interference that
prevents the device or other devices from operating
properly.
2. Plug the charger into a wall outlet and attach the USB
cable to the COMS One power supply and the COMS
One.
3. The battery indicator will indicate charging. During char-
ging the light below the battery symbol will be blinking
green. When the battery is fully charged the battery
indicator will stop blinking and indicate battery full with
a solid green light.
It takes approximately 2 hours to fully charge the device.
When the device is fully charged, it has enough power for at
least 6 treatments. The battery has an expected lifetime of 3
years. After 3 years of intensive usage, it may no longer be
sufcient for 6 treatments with one charge.
Remove the charger from the wall socket and pull the USB
plug out of the USB socket to disconnect device from the
mains supply.
Therefore charger and device must be placed easily access-
ible. Let the device rest 5 minutes after charging.
1
2 3 4
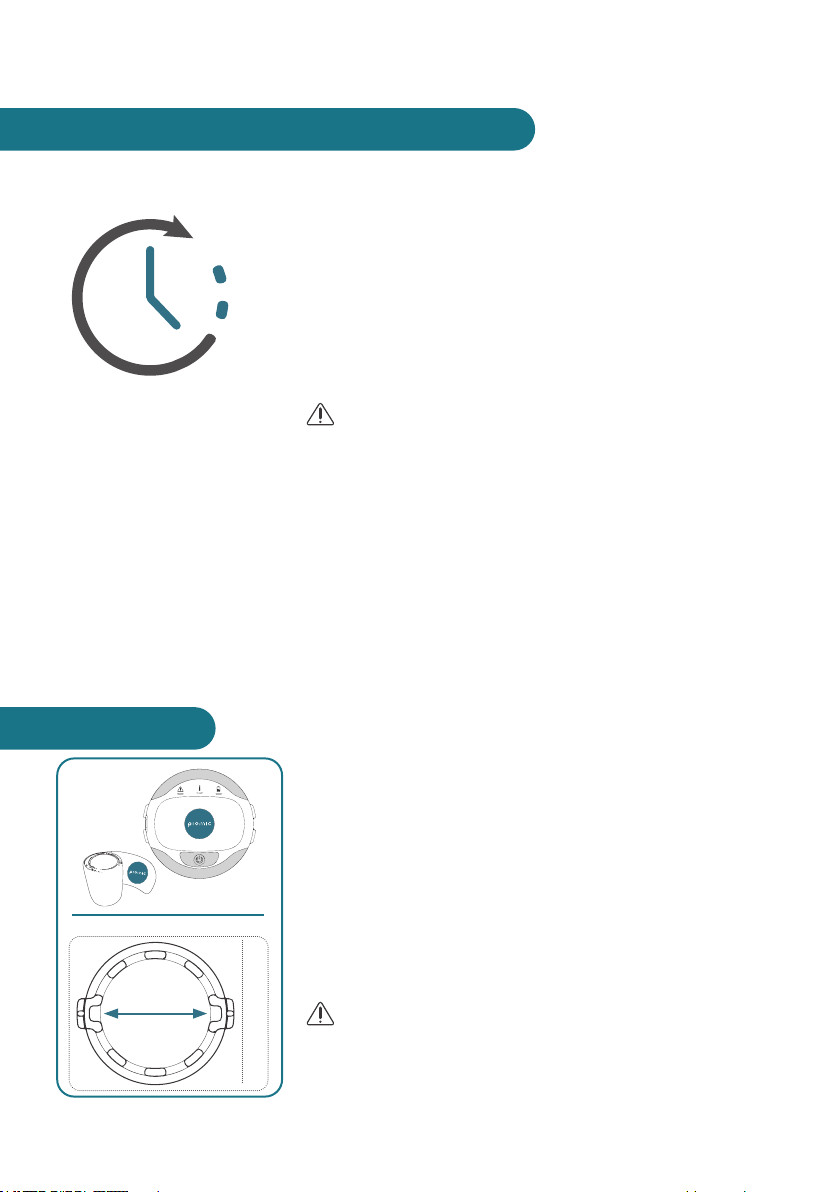
10
X. KAPITELNAME
The COMS One offers a non-invasive and non-toxic
therapeutic approach to promote wound healing. The
system combines the technologies of pulsed electro-
magnetic elds and photon emission applied locally to the
wound area.
A therapy takes 16 minutes. In order to reach the scienti-
cally proven promotion of wound healing the therapy
shall be performed 2-3 times per week and be
repeated over the course of two months.
CAUTION
• Do not apply device more than once within 12 hours on
the same location to prevent overstimulation of the tissue.
Temporary increased exudate production and modied pain
perception cannot generally be excluded for this therapy.
Applicable accompanying wound care measures such as in-
ammation control, pain and exudate management, moisture
control and removal of wound debris must be addressed.
5. THERAPY DURATION AND REPETITION
16 minutes
6. THERAPY
1. Open the COMSx packaging and place strap within
reach of the device.
2. Take a packaged COMStouch and check that maximal
wound size is within the diameter of the COMStouch.
WARNING
• Do not use the COMStouch if the sterile packaging is
damaged or has expired.
8 cm
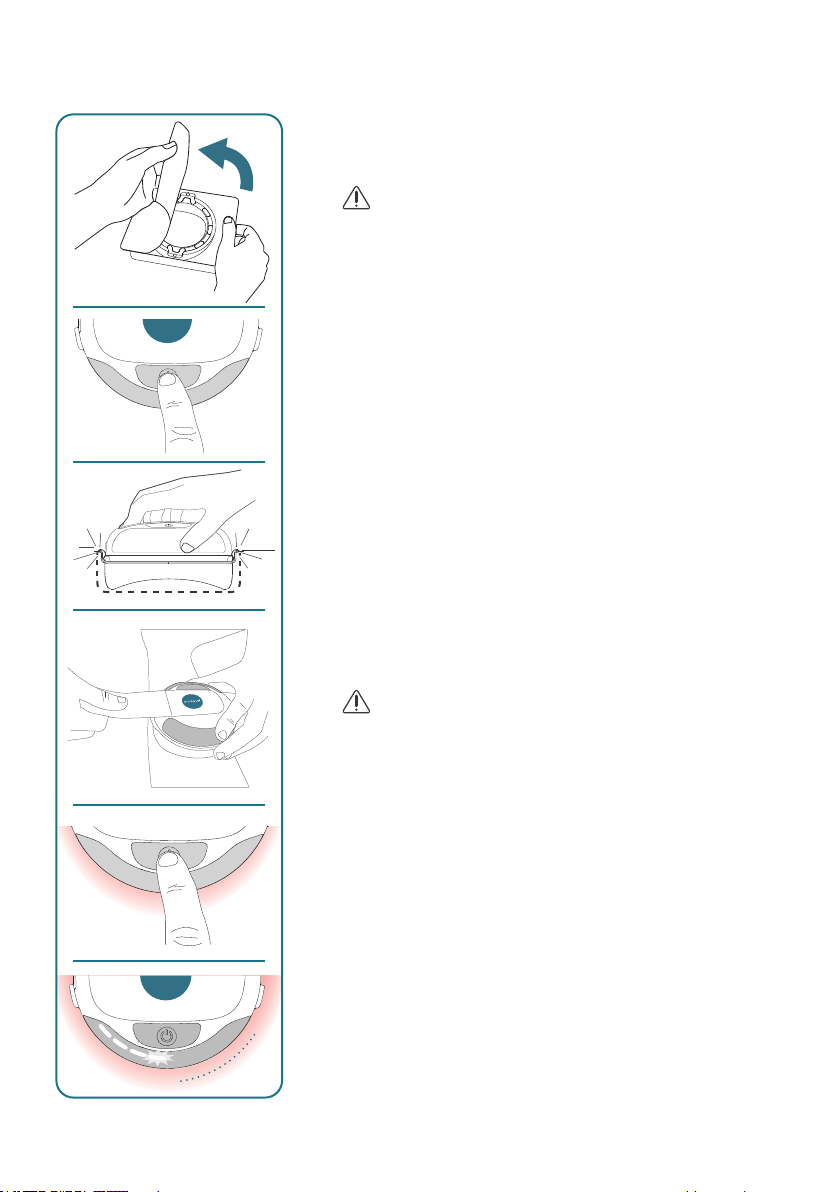
11
ENGLISH
0“2“ 4“ 6“ 8“
3. Carefully open the sterile packaging without touching the
COMStouch.
WARNING
• The COMStouch is for single-use only and cannot be
reused.
4. Take the device and press the On/Off – Button for
longer than 3 seconds to switch the device on.
The device will then do a Power On Self Test. After the
test the device will go into Standby Mode. In Standby
Mode the battery indicator will show if the battery char-
ge level is sufcient for a full therapy (green, proceed
with next step) or insufcient (red, go to chapter “Battery
Charging”).
5. Take the COMS One and mount it to the COMStouch.
The proper xation of the COMStouch to the COMS
One will be indicated by a click-in noise.
6. Carefully mount the device with the attached consu-
mable to the patient using the strap (Loops shall face
towards the device / Logo on Logo).
CAUTION
• Fix the strap carefully to ensure no pressure oedemas
are created.
7. Press On/Off-Button (<1s) to start the therapy
(you will hear a single beep and a red shining on the
bottom side of the device)
To pause therapy, press On/Off-Button anytime (<1s).
8. The therapy progress is indicated by the blinking pro-
gress bars. Each one representing 2 minutes elapsed
time.
CLICK
CLICK
beep
12
X. KAPITELNAME
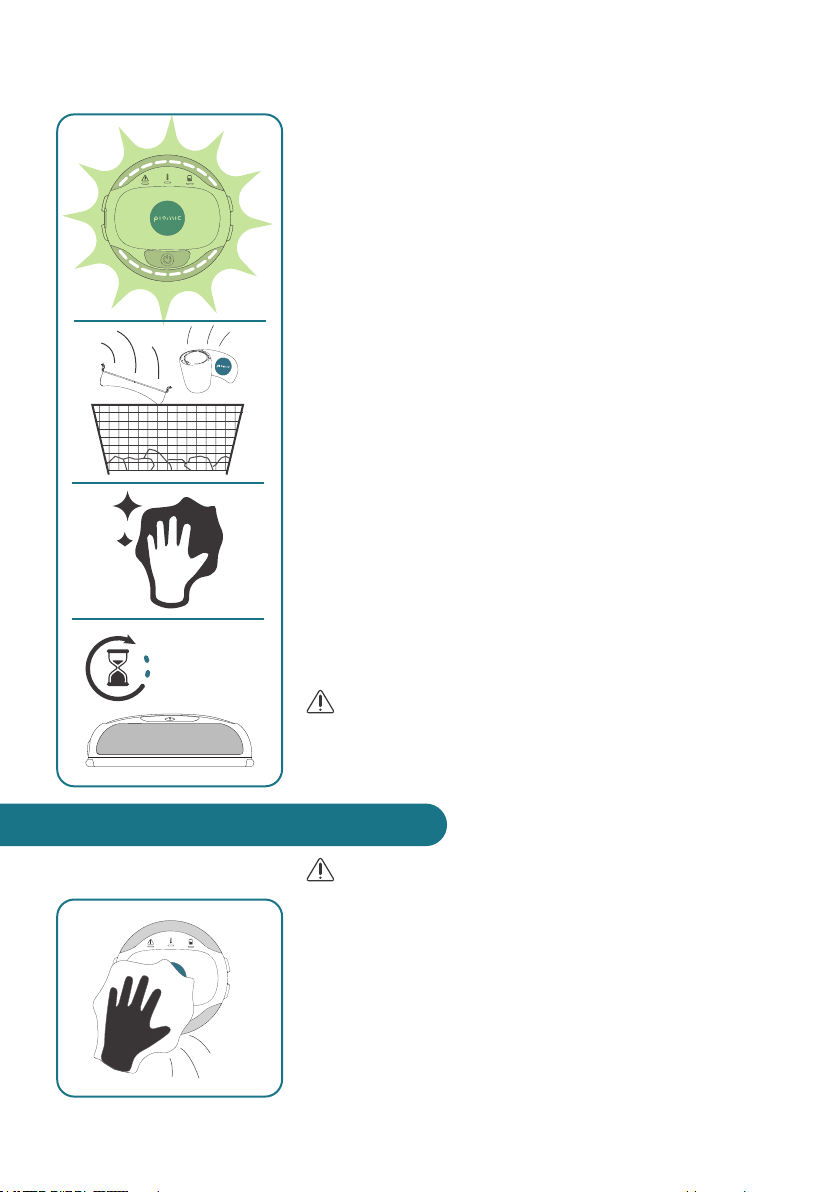
WARNING
• Unplug the device before cleaning and disinfection.
• Do not clean, disinfect or perform other service and
maintenance tasks during use of the device.
The COMS One device is reusable. Thorough cleaning
and disinfection between use on different patients is
important.
1. Cleaning: Wipe off with a clean, damp cloth (water or
non-abrasive detergent).
7. CLEANING AND DISINFECTION
9. Therapy has been completed.
(You will hear 3x beep-sound).
Turn device off by pressing the On/Off button for longer
than 3 seconds.
10. Carefully remove the strap (COMSx/COMSrex)and
COMS One from patient and dispose the single-use
material (COMStouch & COMSx).
11 . Clean and disinfect device before using it on next patient
(see next chapter).
12. Let the device rest before the next use (60min). This
prevents heating of the device at high ambient tempe-
ratures and allows the disinfectant to act optimally.
CAUTION
• Do not apply device more than once within 12 hours on
the same location to prevent overstimulation of the tissue.
60 minutes
13
ENGLISH
8. STATUS INDICATIONS & TROUBLESHOOTING
Indicator Denition Troubleshooting Remarks
Battery good n.a. Blinking during charging until
full (change to static green)
Battery low Charge now or after next
therapy
At least one more therapy
is possible but charging is
recommended
Battery empty Charge now
Charge before next therapy
(Charging for one therapy
takes around 16 min)
Device overheated Cool device before use
Let device cool down before
next therapy. Best to use
another device
Device error Switch the device off and
turn it back on.
If the error persists, contact
Piomic Customer Service
2. Disinfection: Disinfect with wipes from the disinfecting
agent group «alcohol»: E.g. CaviWipes™ (Metrex Rese-
arch, LLC) or Mikrozid® AF (Schülke & Mayr GmbH).
CAUTION
• Do not immerse the device directly in water or other
liquids
Safety related tip
• Do not dry the device with a microwave
• Do only use cleaning and disinfection agents as
described above.
The strap COMSrex is single patient use and may be
cleaned by hand with non-abrasive, standard hand wash
detergents. Air-dry, do not tumble dry or iron.
Store the system in a way that only authorized persons have
access for usage.
%
14
X. KAPITELNAME
Problem Possible Cause Troubleshooting
The charger becomes warm
during charging This is normal No action required
The Battery indicator does not
ash green while charging
The charger is not inserted pro-
perly into the wall outlet
Plug the charger into a wall
outlet properly
“The USB plug is not inserted
properly into the charger
Insert the standard USB plug into
the charger properly
“ The wall socket is not live
Check the wall socket with
another appliance. If the wall
socket is live but the COMS One
does not charge please contact
Piomic Customer Service
The COMS One becomes warm
during use This is normal No action required
The COMS One does not react
to button press Battery is very empty
Please charge the device and try
again. If the error persists, con-
tact Piomic Customer Service
Only 4 light sources are emitting
red light on the bottom side of
the device
This is normal. The other 4 light
sources emit light invisible to the
human eye
No action required.
CAUTION: Do not look
directly into the light source on
the bottom of the device. It may
result in eye injuries
15
ENGLISH
9. SIGNS & SYMBOLS
This symbol indicates a safety
related tip.
To identify the battery
condition.
This symbol indicates a general
warning.
This symbol indicates temperature or
function associated with tempera-
ture.
This symbol indicates standby
(located on On/Off button).
This symbol indicates to
follow the Instructions for Use.
This symbol indicates the date of
manufacture.
This symbol indicates that the device
should not be used after the date
shown.
This symbol indicates the name and
the address of the manufacturer.
This symbol indicates to not use the
device if package is damaged.
This symbol indicates the device is
sterilized using ethylene oxide.
This symbol indicates the number of
items (1 pcs in this case).
This symbol indicates a prescription devi-
ce. CAUTION: U.S. Federal law restricts
this device to sale by or on the order of
a physician (for US only).
This symbol indicates a single use
device. Do not reuse the device.
This symbol indicates a type BF
applied part.
This symbol indicates the
temperature limitation for operation,
transport and storage.
This symbol indicates manufacturer’s
catalogue number.
This symbol indicates the atmo-
spheric pressure limitation for
operation, transport and storage.
This symbol indicates manufacturer’s
serial number.
This symbol indicates the
humidity limitation for operation,
transport and storage.
This symbol indicates manu-
facturer’s batch code. This symbol indicates MR unsafe.
only

16
X. KAPITELNAME
This symbol indicates to handle the
fragile device with care.
This symbol indicates the correct
upright position of the transport
package.
This symbol indicates to keep the
device dry.
This symbol indicates that the device
is in conformance with the Medical
Device Directive 93/42/EEC.
This symbol indicates to keep the
device away from sunlight.
This symbol indicates a general
warning. Refer to chapter 2 warning
and safety instructions.
IP22 This simboly indicates the device is
protected against ingress of solids
larger than 12.5 mm and dripping
water.
This symbol indicates that the mar-
ked item or its material is part of a
recovery or recycling process.
This symbol indicates to not dispose
the device together with household
refuse (for EU only).
This symbol indicates the direct
current socket.
This symbol indicates that the mains
adapter is a class II device.
This symbol indicates alternating
current.
This symbol indicates that the mains
adapter is for indoor use only.
This CE-mark indicates compliance
with the low voltage and electroma-
gnetic compatibility directive.
This symbol indicates the com-
pliance with AUS/NZ regulatory
requirements
This symbol indicates polarity of d.c.
power connector.
This symbol indicates the complian-
ce with energy efciency require-
ments.
This symbol indicates the complian-
ce with USA and Canada safety
requirements.
1250

17
ENGLISH
Device Ref. 01.0000
Optical output
classication
Exempt Group according to IEC 62471:2006. Meaning safe under
reasonably foreseeable conditions.
Power Density
Pulse Peak Power: 25 mW/cm²; Duty cycle: ≈20%;
Average Power: 5 mW/cm²
The local variation across the treatment area between maximum and mini-
mum power density is < factor 2.5
Maximum treatment dose 5 J/cm²
Pulse Specications Maximum pulse width of 0.3 ms at a repetition rate of 1 KHz for 16 min
treatment.
Maximum Spectral
Irradiance
660nm: 5.5 mW/(m² nm)
830nm: 2.2 mW/(m² nm)
10. TECHNICAL SPECIFICATIONS (INCL. WARRANTY AND MAINTENANCE)
The COMS One is certied as medical non-laser light source equipment. While in operation
it generates light in the red and near-infrared spectrum around 660 nm and 830 nm. On the
bottom of the device there are four emission apertures for 660 nm LEDs and 830 nm each.
265 g without Accessories Operating Conditions
113 x 110 x 37 mm Transport/Storage Conditions
(also applicable between uses)
Medical Device Class lla Power Supply
Model S008ACM0500200
Input: 100-240 VAC, 50/60 Hz 300 mA
Output: 5.0 VDC 2.0 A
Device:
[VDC] 5
[W] 7.5
IP 22
Battery (Lithium-Ion)
3.7 VDC
Rated Capacity 3000 mAh
+5
+30
15
90
700
1060
hPa
Specications
700
1060
hPa
-20
+50
20
95
18
X. KAPITELNAME
Warranty
The warranty period for the COMS One is 3 years, if used in
accordance with the Instructions for Use. The manufacturer is
not liable for any damage or consequential damage caused
by incorrect operation, inappropriate usage or unauthorized
persons using the device. The warranty does not cover wear
and tear.
Maintenance
The COMS One is maintenance free and will not require
service. If the device fails within the warranty period due to a
manufacturing defect, the device will be replaced.
The original device will need to be returned to the supplier.
The battery cannot be removed.
The expected service life of the device is 5 years.
Safety-related checks
The COMS One is a Class II electrical appliance. The sa-
fety-related checks are conned to visual inspection of the
device and power supply for damage. These checks must be
performed prior to each use.
Class II electrical appliances do not have a protective earth
conductor. There is no need to check the earth leakage
current.
The COMS One device enclosures are made entirely of
electrically insulating material. Tests of the enclosure leakage
Materials
COMS One Housing Acrylnitril-Butadien-Styrol-Copolymer (ABS), Methylmethacrylate
Acrylnitril-Butadien-Styrol (MABS), Thermoplastic Elastomer (TPE)
COMStouch Silicone, Polybutylenterephthalat (PBT)
Sterilization Method: Ethylene Oxide (EO)
COMSx / COMSrex Polyamid (PA), Elastan, Polypropylen (PP), Synthetic Rubber

19
ENGLISH
current using common measuring instruments will therefore not
reveal measurable values.
The device does not have patient circuits or functional earth
connections.
Storage to therapy time
Note that if you store the product at very low temperature
(below +5 degree of Celsius) or very high temperature
(>30 degree Celsius) it may take up to 30 minutes to reach
operating temperature.
Disposal
COMS One comprises metals and plastics and should be
disposed of in accordance with the European directives
2011/65/EU and 2012/19/EU. The electronic componen-
ts must be disposed of separately, in accordance with the
local regulations. This product contains a lithium-ion battery
which bear risk of re, explosion and burns, if disposed of
improperly. Please take care that you dispose of COMS One
and its accessories in accordance with the local regulations
and applicable disposal guidelines.
Do not throw away the device with the normal household
waste at the end of its life, but hand it in at an ofcial col-
lection point for recycling. By doing this, you help to preserve
the environment. This symbol is only valid in the European
Union. Please respect the relevant state laws and rules in your
country for the disposal of electrical and electronic equip-
Manufacturer’s legal address
Piomic Medical AG
Reitergasse 6
8004 Zürich
Switzerland
www.piomic.com
Tel.: +41 44 244 19 70
Please contact the manufacturer in case you encounter or
detect any abnormalities with the device.
Table of contents
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual











