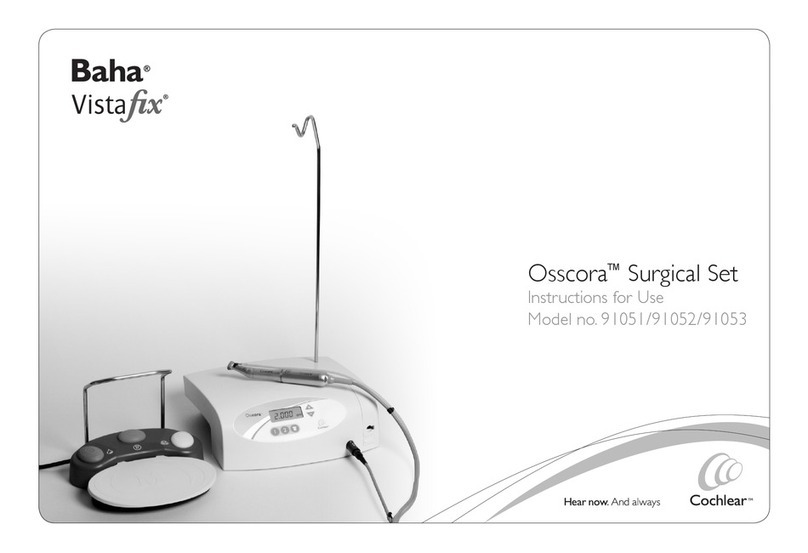
Primedic HeartSave 6 User manual

www.primedic.de
O
p
erating Instructions
21230 / GB / F02

21230 / GB / F02
Masthead
Publisher
METRAX GmbH
Rheinwaldstr. 22
D-78628 Rottweil
Germany
Tel.: + 49 (0)741 257-0
Fax.: + 49 (0)741 257-235
www.primedic.com
by METRAX GmbH
These operating instructions may not be reproduced, transferred, electronically saved or
translated into a human or computer language in whole or in part without our permission.
Violations of this prohibition not only infringe our copyright, but also reduce our possibilities to
provide the users and operators of the device with precise and punctual information.
We reserve the right to make amendments to these operating instructions.
Printed in Germany

Table of contents
I
Table of contents
1 Introduction 2
1.1 Foreword.....................................................................................................................2
1.2 Validity........................................................................................................................2
1.3 Warranty.....................................................................................................................2
1.4 Disclaimers..................................................................................................................3
1.5 Symbols used in these operating instructions ...............................................................3
1.6 Pictograms on the device.............................................................................................4
1.7 Pictograms on SavePads..............................................................................................5
2 Intended use 6
2.1 Indications...................................................................................................................7
2.2 Contraindications ........................................................................................................7
3 Safety advice 8
3.1 General advice.............................................................................................................8
3.2 General safety advice...................................................................................................9
3.3 Safety advice for you, the user.....................................................................................9
3.4 Safety advice for protection of the patient.................................................................10
3.5 Safety advice for the protection of third parties..........................................................11
3.6 Safety advice for the protection of the device............................................................11
4 Description of device 12
4.1 General description....................................................................................................12
4.2 Description of device details ......................................................................................13
4.3 Icons in the status display..........................................................................................17
4.4 Capacity display ........................................................................................................18
4.5 Data management.....................................................................................................19
5 Description of the accessories 20
5.1 SavePads...................................................................................................................20
5.2 Two-pin patient cable for ECG recording (optional accessory)....................................20
5.3 Three-pin patient cable for ECG recording.................................................................21
5.4 SpO2sensor (only on HeartSave 6S)...........................................................................21
6 Preparatory measures before (initial) start-up 22
6.1 Unpacking.................................................................................................................22
6.2 Inserting / Replacing the SaveCard............................................................................22
6.2.1 Inserting the SaveCard ...................................................................................23
6.2.2 Replacing the SaveCard..................................................................................23
6.3 Inserting / replacing the energy module.....................................................................23
6.3.1 Inserting the power module............................................................................24
6.3.2 Removing the power module from the device ................................................25
6.4 PRIMEDIC™ battery (optional)..................................................................................26
6.5 PRIMEDIC™ AkuPak.................................................................................................26
6.6 Charging the AkuPak with the PRIMEDICTM ClipCharger............................................28
6.7 Charging the AkuPak in the optional PRIMEDIC™ Charger Basis / Charger Comfort.29
6.8 Connecting up the PRIMEDIC TM PowerLine (optional accessory) ...............................29
6.9 Periodic device self-test .............................................................................................30
6.9.1 Self-test after switching the HeartSave on ......................................................30
6.9.2 Periodic self-test.............................................................................................30
7 Using the device 31
7.1 Switching on/off .......................................................................................................31
7.1.1 Switching the PRIMEDIC™ HeartSave 6/6S on ..............................................31
7.1.2 Switching the PRIMEDIC™ HeartSave 6/6S off..............................................31
7.2 Selecting the operating mode....................................................................................31
7.2.1 Automatic mode (AUTO mode) .....................................................................31
7.2.2 Manual mode (Auto Sync)..............................................................................32
7.2.3 Changing the operating mode........................................................................32

Table of contents
21230 / GB / F02
7.3 Setup menu ..............................................................................................................33
7.3.1 Simple change to configuration – example: Time ...........................................34
7.3.2 Changing the PIN..........................................................................................35
7.3.3 Calling up/activating a profile........................................................................35
7.3.4 Saving menu parameters in a single profile.....................................................36
7.4 Alarms......................................................................................................................37
7.4.1 ECG Alarm.....................................................................................................37
7.4.2 VF Alarm.......................................................................................................37
7.4.3 SPO2 Alarm....................................................................................................38
8 Positioning the electrodes 39
8.1 Undressing the patient..............................................................................................39
8.2 Positioning the defibrillation electrodes (SavePads)....................................................39
8.3 Checking the skin......................................................................................................40
8.4 Positioning the ECG adhesive electrodes ...................................................................40
8.5 Connecting the electrodes.........................................................................................41
8.6 < Plug in electrode plugs >........................................................................................42
8.7 Check electrodes.......................................................................................................42
9 Operation in Automatic Mode 43
9.1 Voice prompts by the device/Preliminary examination of the patient(ERC)................43
9.2 Performing the ECG analysis in Auto Mode...............................................................43
9.3 Defibrillation required ...............................................................................................44
9.4 Defibrillation not required .........................................................................................45
9.5 Keeping the defibrillator ready for use.......................................................................46
10 Operation in Manual Mode 46
10.1 Performing defibrillation............................................................................................46
10.2 AUTO-SYNC.............................................................................................................48
10.3 Keeping the defibrillator ready for use.......................................................................49
11 Attaching the SpO2sensor 49
11.1 Connecting the SpO2 sensor.....................................................................................50
12 Cleaning, maintenance and dispatch 51
12.1 Cleaning ...................................................................................................................51
12.2 Servicing...................................................................................................................51
12.2.1 Servicing check list.........................................................................................51
12.3 Dispatching the PRIMEDIC™ HeartSave ...................................................................52
12.4 Disposal....................................................................................................................52
13 Technical Data 53
14 Warranty conditions 56
15 Depiction of the current-time function 57
16 Rhythm detection system 61
17 General advice for using pulse oximeters 64
18 Guidelines and manufacturer's declaration – Electromagnetic emissions 66
19 General instructions and rules for using the optional AkuPak 70
20 Safety checks 72
21 Using the equipment on ships 73
21.1 Use of PRIMEDIC™ HeartSave units together with a PRIMEDIC™ battery on ships in
the merchant navy:............................................................................................................73
21.2 Use of PRIMEDIC™ HeartSave units together with a PRIMEDIC™ AkuPak on ships in
the merchant navy:............................................................................................................73

Table of contents
III
Contact details 74

Introduction
Operating Instructions HeartSave 6 / 6S, 21230 / GB / F02
2
1Introduction
1.1 Foreword
Dear User,
You are faced with the task of using the PRIMEDIC™ HeartSave in a medical
emergency on human beings.
So that you react quickly and properly in these special circumstances and make optimal
use of the opportunity the device provides you with , it is necessary for you to take
your time and read through these operating instructions beforehand, thus familiarising
yourself with the device, its functions and applications.
Keep these operating instructions near the device so that you consult any queries
which may arise.
If you have any questions regarding the device or other PRIMEDIC™ products, we
would be glad to be at your disposal.
You will find our contact address on the masthead at the start of these operating
instructions.
1.2 Validity
This operating manual describes the
HeartSave 6 and HeartSave 6S defibrillators supplied by METRAX GmbH.
1.3 Warranty
The warranty period is 24 months and starts on the day of purchase. Please keep the
invoice as proof of purchase.
The general guarantee and warranty provisions of METRAX GmbH are applicable.
Any repairs or changes to the device may only be carried out by the manufacturer or
by a person or company authorised by the manufacturer.
Introduction
GB HeartSave 6 / 6S, 21230 / GB / F02
3
1.4 Disclaimers
Liability claims in the event of damages to people or property are excluded if they are
based on one or more of the following reasons:
Using the device in a manner for which it was not intended.
Improper use and maintenance of the device.
Operating the device with the protective covers removed or when there is obvious
damage to cables and/or electrodes.
Non-compliance with the instructions in these operating instructions with regard
to operation, maintenance and repair of the equipment.
Using accessories and spare parts made by other manufacturers.
Autonomous intervention, repairs or constructional changes to the device.
Autonomous overrunning of the performance limits.
Lack of monitoring parts that are subject to wear and tear.
Treatment of patients without prior indication.
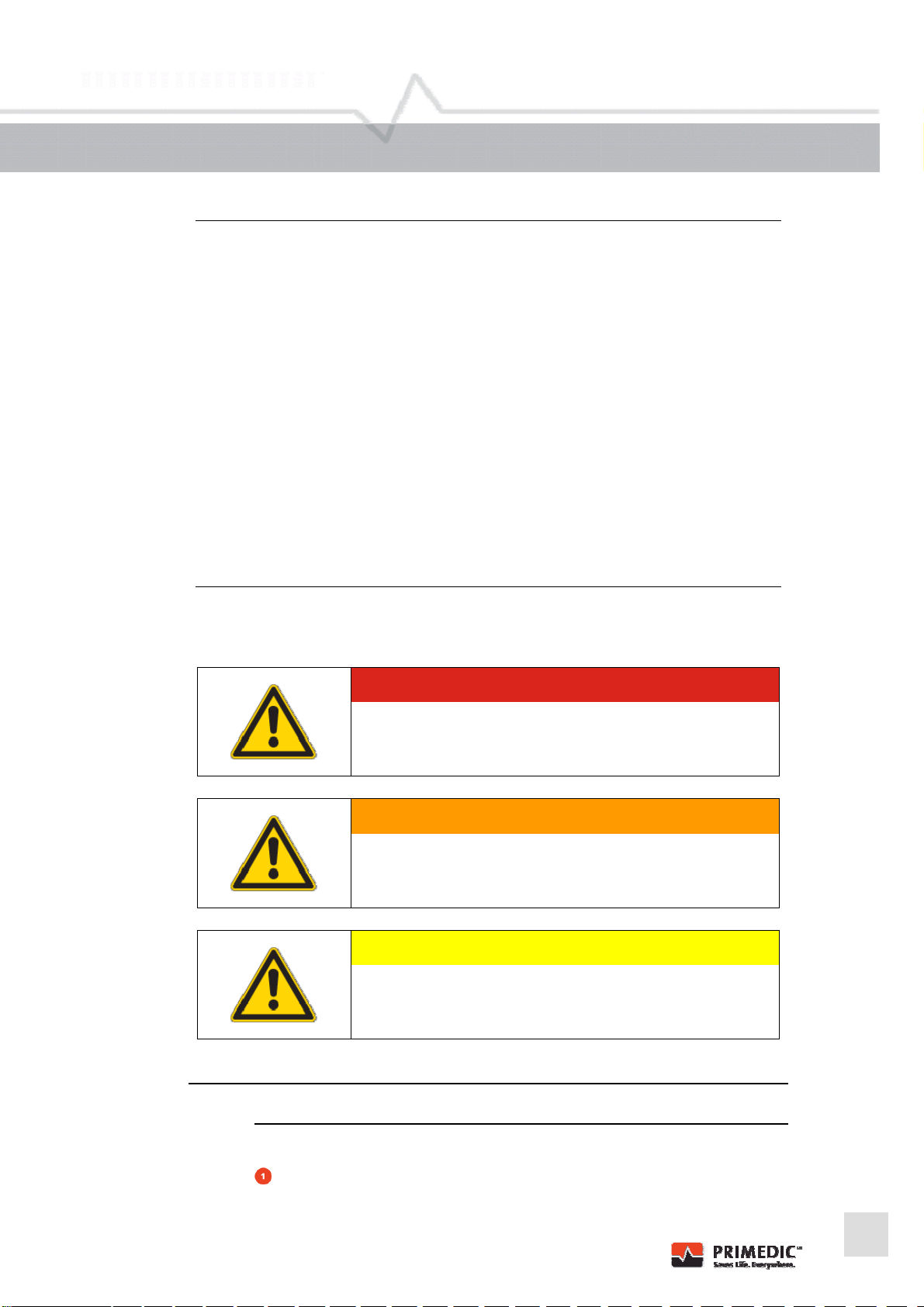
1.5 Symbols used in these operating instructions
DANGER
Texts marked DANGER indicate an extraordinarily serious,
current danger which will definitely lead to serious injury or
even death if no preventative measures are adopted.
It is imperative that you pay attention to these texts.
WARNING
Texts marked WARNING indicate an extraordinarily serious,
potential danger which could lead to serious injury or even
death if no preventative measures are adopted.
It is imperative that you pay attention to these texts.
CAUTION
Texts marked with CAUTION indicate a possibly dangerous
situation which could lead to minor injuries or damage to
property.
It is imperative that you pay attention to these texts.
Note This symbol indicates text which contains important advice / comments or
tips.
This point identifies the description of tasks that you need to perform.

Introduction
Operating Instructions HeartSave 6 / 6S, 21230 / GB / F02
4
This point identifies a list.
(3) Numbers in brackets refer to items in diagrams.
< ... > Texts set in pointed brackets denote acoustic advice/instructions by the
device, which are also shown simultaneously on the monitor (only on
HS6).
1.6 Pictograms on the device
IP55
IP53
IP33
Protection against contact and dust deposits on the inside
Protection against jets of water (nozzle) from any angle
(only in combination with a battery)
IP53 in connection with a battery pack
IP33 in connection with PowerLine
Please observe the operating instructions.
Do not dispose of device in domestic refuse.
Dangerous electric voltage (high voltage)
CF defibrillation-proof degree of protection, in connection with ECG patient cable
Type certification GERMANISCHER LLOYD
in accordance with Certificate No. 75 449-09 HH

Introduction
GB HeartSave 6 / 6S, 21230 / GB / F02
5
1.7 Pictograms on SavePads
Not to be reused
Expiry date
Batch ID
Only for adults
Order number
Storage temperature information
in Celsius and Fahrenheit

Intended use
Operating Instructions HeartSave 6 / 6S, 21230 / GB / F02
6
2Intended use
The HeartSave is designed for use in automatic mode by suitably qualified first-aiders,
trained paramedics and doctors in their everyday clinical activities, either within a
hospital or in the preclinical area of emergency medicine. Only doctors are authorised
to use the device in manual mode. It may only be used on patients who are
unconscious and who are not breathing. The device may be powered from different
removable power modules. Thanks to its compact, light design, the HeartSave can
easily be transported in an ambulance with a patient.
The device is used to carry out transthoracic defibrillations. The main application is
defibrillation in asynchronous manual mode; an additional application is the
cardioversion for atrial fibrillation in synchronous manual mode. The decision on
whether delivery of a shock is required can be made either by the user in manual
mode, or automatically with the shock recommendation from the device in AED mode.
In automatic mode, the energy levels for the first, second and third shock are specified
by the maximum voltage setpoint values of 20A, 25A and 30A, and by the capacitor
voltage determined by the patient impedance; while in manual mode, you can select
energy levels between 50- 360J to adjust the defibrillation energy appropriately in line
with weight and the doctor's experience.
The device is also used to record and display electrocardiograms (monitoring). The
derivation from the defibrillation electrodes is calculated in compliance with correct use
of the electrodes from the Einthoven II derivation. Dual-channel monitoring is possible
if you use an ECG cable instead of defibrillation electrodes and commercially available
ECG electrodes. A random (appropriate) selection of 2 signals from the Einthoven I, II,
III or Goldberger aVR, aVL, aVF – analogue derivations can be displayed. Correct
positioning is mandatory.
The operator is guided by spoken instructions and clear written and pictorial
information. After switching on the device, the patient is connected to it using the
enclosed adhesive electrodes. After this, automatic rhythm analysis is carried out by the
device. Only if a rhythm is detected by the device as being ventricular fibrillation
(=requiring a shock) does it suggest treatment with a high energy shock. All other
rhythms are classified as not requiring a shock. The time from the start of analysis until
the shock is applied is less than 30 s.
For safety reasons, no shock is given with asystolia, as no therapeutic effect is to be
expected. Controlled ventricular electrical activity caused by supraventricular
tachycardia such as atrial fibrillation, atrial flutter, ventricular extra-systoles and
idioventricular rhythms does not lead to a shock being applied. Following application of
the shock, the device carries out a new rhythm analysis. Additional shocks are
recommended if other rhythms (ventricular fibrillation) are present that can be treated
with a high-energy shock. The user performs resuscitation using manual cardio
pulmonary resuscitation, in accordance with the applicable guidelines.
Any use above or beyond this is not considered as intended use and can lead to
personal injury or damage to property.
Improper use of the defibrillator can lead to ventricular fibrillation, asystolia or other
dangerous dysrhythmia.
The operator of the HeartSave must ensure that the HeartSave is only used by
authorised specialist personnel.

Intended use
GB HeartSave 6 / 6S, 21230 / GB / F02
7
General note:
The guidelines governing the application of emergency treatment in the event of
cardiac arrest may change. The current device can be operated either on the basis of
the International Guidelines 2005 Resuscitation (2005) 67S1, S7—S23 by the European
Resuscitation Council or on the basis of the American Heart Association (AHA)
guidelines for cardiopulmonary resuscitation (CPR) 2005.
The PRIMEDIC™ HeartSave may only be used as described and under the conditions
detailed in these operating instructions.
2.1 Indications
The PRIMEDIC™ HeartSave 6/6S may only be used for defibrillation if the patient
is unconscious and
no normal breathing can be ascertained; and
after talking to the patient, no other signs of life can be perceived.
2.2 Contraindications
The PRIMEDIC™ HeartSave 6/6S must not be used for defibrillation if the patient
is conscious; or
breathing; or
shows other signs of life
is a child under the age of 8 or weighs less than 25 kg respectively.
Treatment should not be delayed to ascertain the precise age or weight of the
patient.

Safety advice
Operating Instructions HeartSave 6 / 6S, 21230 / GB / F02
8
3Safety advice
3.1 General advice
The PRIMEDIC™ HeartSave 6/6S fulfils the currently applicable safety standards and
complies with the provisions of the medical products guidelines, both as a stand-alone
device and in conjunction with its fittings and optional accessories.
The device and its accessories are safe when used as intended and when following the
descriptions and information detailed in these operating instructions.
Nevertheless, if used incorrectly, the device and its accessories can be dangerous for
the patient or third parties.
DANGER
We emphatically advise that before using the device for the
first time, all those who are supposed to use or want to use
it
must be instructed in a training session about the
medical back
g
round of defibrillation and the indications
or contraindications and thus need to be authorised.
need to read and take note of these operating
instructions and in particular the safety tips and
warning advice detailed in them.
CAUTION
The PRIMEDIC™ HeartSave 6/6S may only be used by
trained and authorised personnel. Reading the operating
instructions does not replace training.
The PRIMEDIC™ HeartSave 6/6S is not licensed for use in
explosive areas.
DANGER
Not using the device as intended or using it improperly,
exposes the user, the patient or third parties to the danger
of an electric shock from the high voltage generated by
the device,
of influencing active implants,
of burns from incorrectly applied electrodes.
Apart from that, the device can be damaged or destroyed
through improper use.
Refer to the advice and rules in the appendix when using the PRIMEDIC™ HeartSave
6/6S.

Safety advice
GB HeartSave 6 / 6S, 21230 / GB / F02
9
Applicable for Europe:
The device complies with the Medical Device Directive (MDD).
For Germany, the following also applies:
The device complies with the Medical Devices Law (MPG) and is subject to the
Ordinance on the Operation and Use of Medical Devices (MPBetreibV).
According to the Ordinance on the Operation and Use of Medical Devices
(MPBetreibV), the device must be subjected to the regular checks explained in the
appendix.
According to the Ordinance on the Operation and Use of Medical Devices
(MPBetreibV), a medical devices log needs to be kept for the device. Regular checks of
the device are to be documented in it.
For the other states in the European Community, national regulations for operating
medical devices apply.
3.2 General safety advice
DANGER
You must not use the device in the vicinity of flammable
materials (e.g. cleaning solvents or similar) or in an
atmosphere enriched with oxygen or flammable
gases/vapours.
3.3 Safety advice for you, the user
WARNING
Only use the device on a patient if
you have ensured its operational safety before use and
are certain that the device is in good condition.
the patient's condition requires or permits its
application!
Before using the device, check whether it is in the operating
temperature range. This applies for example, if the
defibrillator is stored in a rescue vehicle.
Do not use the device if it is defective (e.g. if the
defibrillation cable is damaged).

Safety advice
Operating Instructions HeartSave 6 / 6S, 21230 / GB / F02
10
3.4 Safety advice for protection of the patient
DANGER
●Use the unit on a patient only if you have made sure that
functional safety and proper condition of the unit are present!
●Before using the device, check whether it is in the operating
temperature range. This applies for example, if the defibrillator
is stored in a rescue vehicle in the winter.
●Do not use the device if it is defective (e.g. if the defibrillation
cable is damaged).
●Only use the device with accessories, wearing parts and
disposable items which have proven to be completely safe to
use by being tested by a testing authority licensed to test the
device when equipped ready for use.These conditions are
fulfilled by all original PRIMEDIC™ accessories and wear
parts.
●Use new, undamaged defibrillation electrodes before their
expiry date for every patient to avoid any possible burns to the
skin!
●Connect the adhesive electrodes only using the PRIMEDIC™
HeartSave 6 / 6S. Using the electrode system with other
devices may result in the release of dangerous leakage
currents on the patient.
●Do not use the device in the immediate vicinity of other
sensitive equipment (e.g. measuring equipment that is
sensitive to magnetic fields) or strong sources of interference
which could affect the way the PRIMEDIC™ HeartSave 6 / 6S
works. Keep an adequate distance from other therapeutic and
diagnostic energy sources (e.g. diathermy, high frequency
surgery, magnetic resonance tomography).
These devices can affect the PRIMEDIC™ HeartSave 6/6S and
disrupt the way it operates. For this reason, disconnect the
patient from the interfering devices. During defibrillation,
disconnect the patient from all other medically used devices
which do not have a defibrillation resistance application
section. Keep the defibrillation electrodes away from other
electrodes and from metallic parts which are in contact with
the patient.
●Do not use the device on children under the age of 8 or on
children with an estimated body weight of less than 25 kg!
●Position the electrodes precisely according to the description.
●Dry the chest and carefully remove any large amount of hair on
the patient before applying the defibrillation electrodes.

Safety advice
GB HeartSave 6 / 6S, 21230 / GB / F02
11
DANGER
•Do not touch the patient during the ECG analysis and avoid
any vibration!.
•Do not place the defibrillation electrodes directly over an
implanted pacemaker to avoid a possible misinterpretation
by the device and to avoid any damage to the pacemaker
from the defibrillation impulse!
•If the ECG analysis is being carried out in a vehicle, the
vehicle has to stop and switch off the engine to guarantee
correct analysis.
• Interrupt the reanimation as long as the PRIMEDIC™
HeartSave 6 / 6S is analysing the ECG
• Do not touch the patient during defibrillation! Avoid any
contact between
p
arts of the
p
atient's bod
y
(such as bare skin on head or
legs), and
conductive liquids (such as gels, blood or salt solutions)
and
metallic objects around the patient (such as bed frame
or bedside stretching aid) that create unintended paths
for the defibrillation current!
3.5 Safety advice for the protection of third parties
DANGER
Give loud and clear warning to people in the vicinity before
the defibrillation to stand clear of the patient and have no
contact with the patient.
3.6 Safety advice for the protection of the device
CAUTION
Repairs, changes, extensions and installation of the
PRIMEDIC™ HeartSave 6/6S may only be carried out by
personnel authorised and trained by METRAX The
PRIMEDIC™ HeartSave 6/6S does not have any parts that
can be repaired by the user.
The device ma
y
onl
y
be e
q
ui
pp
ed and o
p
erated with ori
g
inal
accessories from PRIMEDIC™.
Clean the device only when it is switched off and the
electrodes are unplugged.

Description of device
Operating Instructions HeartSave 6 / 6S, 21230 / GB / F02
12
4Description of device
4.1 General description
The PRIMEDIC™ HeartSave 6/6S is an automatic external defibrillator, with an
integrated 6-channel ECG and manual defibrillation mode.
The ECG can be recorded either using the PRIMEDIC™ SavePads or the three-pin
patient cable.
In automatic mode (Auto Mode), the ECG is analysed with the implemented
algorithm. If potentially fatal cardiac arrhythmia is detected, the device generates the
electrical shock required to resuscitate the patient and recommends defibrillation. An
electrical shock is not generated if the device does not detect a rhythm requiring
defibrillation.
In manual mode, either the doctor or the user decides whether
defibrillation is necessary.
The family of devices is set up on a modular basis. types of models:
HeartSave 6 basic model with monitor and 6-channel ECG
HeartSave 6S basic model with monitor and 6-channel ECG and pulse oximetry
The PRIMEDIC™ HeartSave generation has been designed for rapid and safe use in
emergency situations. All functional units and operating elements are subject to the
following principles:
Clear organisation of functional units
Reduction of functions to those necessary
Intuitive and logical operator guidance
Clear, self-explanatory operating elements
Ergonomic layout.
The ECG monitor has a high-resolution graphical display that delivers high image
contrast even under difficult light conditions.
The defibrillator unit has been optimised to be safe and quickly ready to use. The
loading time for a defibrillation is max. 12 seconds with a battery capacity of approx.
90% of the rated value.
Power is supplied to the PRIMEDIC™ HeartSave 6/6S either by single-use lithium
batteries or by rechargeable removable batteries with nickel-cadmium cells, or via a
mains power unit, depending on the particular model.
Note The wall bracket and accessories are described in separate operating
instructions.

Description of device
GB HeartSave 6 / 6S, 21230 / GB / F02
13
4.2 Description of device details
Fig. 1: PRIMEDIC™ HeartSave 6/6S front view
1 Carry handle
2 Flap for removing the device cover
3 Device cover
Fig. 2: PRIMEDIC™ HeartSave 6/6S rear view
1 Mounting slot for wall bracket
2 Name plate

Description of device
Operating Instructions HeartSave 6 / 6S, 21230 / GB / F02
14
Fig. 3: PRIMEDIC™ HeartSave 6/6S bottom view
1 Release button for removing the power module
2 Power module
Fig. 4: PRIMEDIC™ HeartSave 6/6S control elements
1 On/Off button
2 Membrane keyboard with monitor
3 Key for scrolling upwards in the menu or for increasing parameters
4 Select/Confirm key (Enter key)
5 Key for scrolling downwards in the menu or for reducing parameters
6 Loudspeaker
7 Socket for electrode cable
8 Energy loading button (charging button in manual mode)
9 Socket for SpO2sensor (on HeartSave 6S)
10 Shock button

Description of device
GB HeartSave 6 / 6S, 21230 / GB / F02
15
Fig. 5 Monitor representation
1 Display of switch-on duration / time sequence HLW cycle
2 Mode: MAN = asynchronous, Sync = synchronous, VF Auto = AED
3 EKG channel line
4 Indicator heart rate and alarm limits
5 Indicator Pulsoximeter and alarm limits
6 Status line to show CF card capacity, patient impedance, time of day, microphone,
battery capacity
7 Indicator ECG-channels (max.2 )
8 Energy stages (only in manual mode)
9 SpO2curve (only HeartSave 6S), instructions, information (only HeartSave 6)
Fig. 6: PRIMEDIC™ HeartSave 6/6S status display
1 Status display

Description of device
Operating Instructions HeartSave 6 / 6S, 21230 / GB / F02
16
Fig. 7: PRIMEDIC™ HeartSave 6/6S device cover with kit holder
1 Device cover
2 Kit holder with quick start guide, face shield and razor
3 SavePads
4 Rubber gloves
This manual suits for next models
1
Table of contents
Other Primedic Medical Equipment manuals

Primedic
Primedic DefiMonitor XD User manual

Primedic
Primedic RECK MOTOmed loop User manual

Primedic
Primedic Defi-Monitor ECO 1 User manual

Primedic
Primedic DefiMonitor XD Series User manual

Primedic
Primedic DefiMonitor XD User manual

Primedic
Primedic HeartSave User manual

Primedic
Primedic HeartSave AED User manual

Primedic
Primedic HeartSave AED User manual