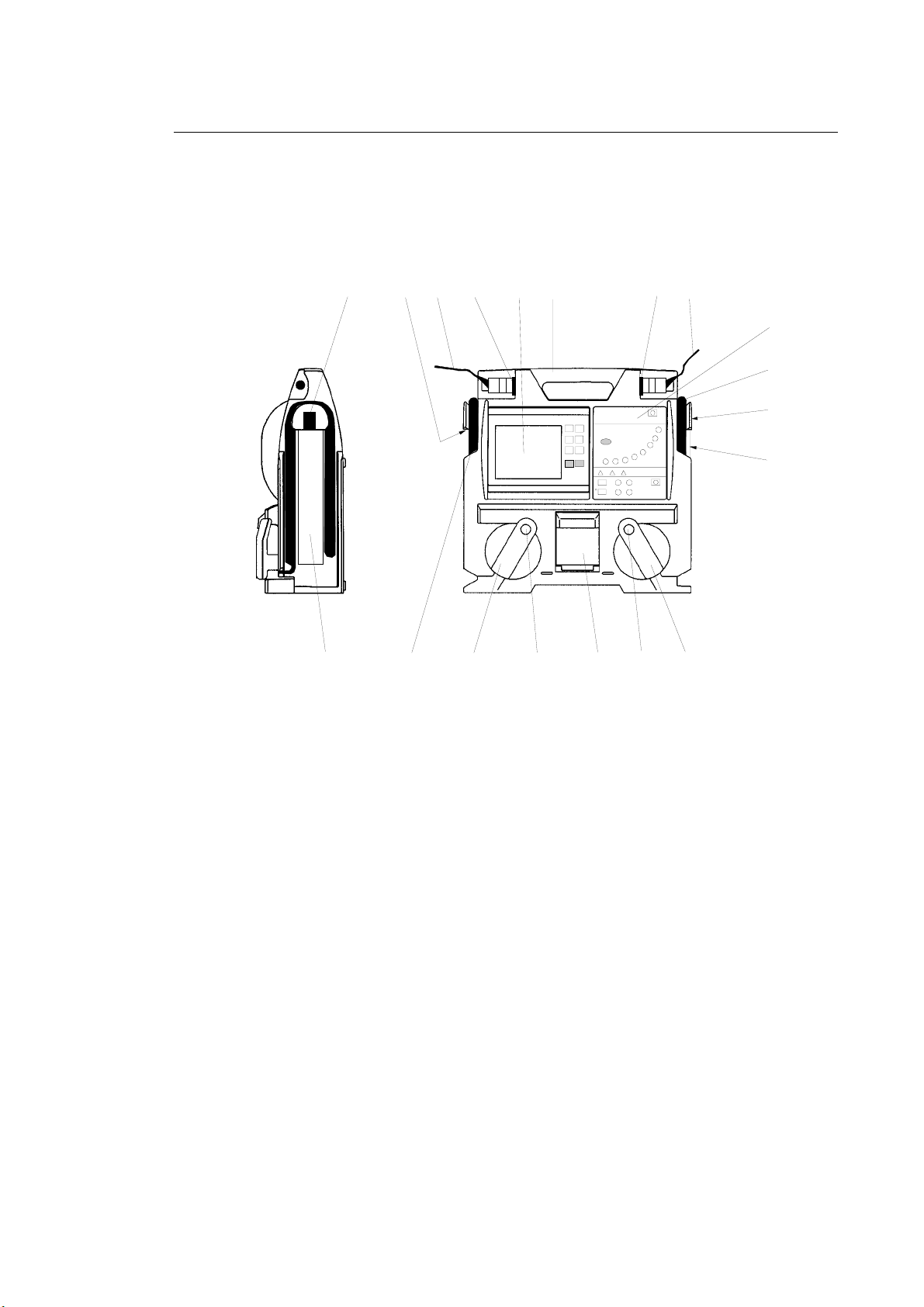
Instructions for use PRIMEDIC™Defi-Monitor ECO 1 / DM 1 / DM 3
19499 / 05.03 61
1. Safety instructions
Your PRIMEDIC™Defi-Monitor was designed in accordance with the high requirements of the use
in emergency situations. Modern technology based on the many years of experience in the
development and production of defibrillators together with new shock absorbing materials and new
ideas offer the necessary support when every second counts.
The following has to be considered in order to ensure safe and perfect function of the PRIMEDIC™
Defi-Monitor and to avoid risk to human beings and other material property:
1. Any use of the PRIMEDIC™Defi-Monitor requires the knowledge and strict compliance of
these instructions for use.
2. The PRIMEDIC™Defi-Monitor is designed and suitable exclusively for the applications set
out or described in this manual. Using the device for purposes any other than those mentioned
in this manual may constitute a risk and has to be omitted.
3. Operation of the PRIMEDIC™Defi-Monitor, as well as basically all other defibrillators, in
areas subject to explosion hazards is not allowed.
4. The PRIMEDIC™Defi-Monitor may only be used by trained and authorised personnel.
Reading the instructions for use does not replace any training.
5. Improper use or excessive operating duration of the SpO2sensor can cause tissue damages.
6. Strong light, movements and certain physical conditions of the patient, intravascular dye,
incorrect attaching of the SpO2sensor could also result in wrong indications of oxygen
saturation. Do not use the SpO2measure as single method to monitor the vital functions.
The instructions for the use of pulsoximeters have to be complied with (appendix A3).
7. Any repair work, modifications, additions and installations of the PRIMEDIC™Defi-Monitor
may only be carried out by personnel authorised and trained by METRAX. The parts of the
PRIMEDIC™Defi-Monitor may not be repaired by the user.
8. The device may only be used with accessories, wearing parts and disposable parts the secure
use of which is proofed by an inspection office authorised to tests of devices ready-to-use.
Otherwise a safe and reliable function of PRIMEDIC™Defi-Monitor is not guaranteed. The
original PRIMEDIC™accessories and wearing parts comply with this condition.
9. Before using the device the user has to check that the device is in a safe and reliable state.
If e.g. the pacer / defibrillator cable is damaged the defibrillator / pacer may not be used.
10. The instructions and rules set out in appendix A1, A2 and A3 have to be complied with when
using the PRIMEDIC™Defi-Monitor.
11. The unit must be under operating conditions before using. This is e.g. essential when storing
the defibrillator in an ambulance car during winter.
12. Do not use the PRIMEDIC™Defi-Monitor near devices (e.g. measuring devices) sensible to
magnetic fields or disturbing sources, which could interfere with the functions of
PRIMEDIC™Defi-Monitor. Keep sufficient distance.
For the other states of the European Union the national regulations for the use of medical devices are
applicable.
Manufacturer: METRAX GmbH
Rheinwaldstraße 22
D-78628 Rottweil
Phone ++49 (0)741 / 257-0