Prism Healthcare harvest healthcare Cadence Profiling Beds User manual

Cadence Profiling
Beds
Last update 02.03.2023
INSTRUCTION
MANUAL
ASSEMBLY AND OPERATION


5. Assembly and commissioning
5.1 Assembly of Erector with triangle handle (accessory)
5.2 Mounting the bed extension (Accessories)
5.3 Protector (Accessories)
5.4 Assembly of the 2-part split aluminum side rails (Accessories)
5.5 Assembly of the 3-part split steel side rails (Accessories)
5.6 Commissioning
6. Functional description
2
5
models 6
7
7
8
10
10
10
11
11
12
12
13
13
14
1. General information
1.1 Explanation of the symbols used
1.2 Explanation of the designated groups of persons
2. Intended purpose
2.1 Intended use (application environment)
2.2 Unauthorised use
3. Safety instructions
3.1 General safety instruction
3.2 Safety instructions for the operator
3.3 Safety instructions for the user
3.4 Cleaning and disinfection
3.5 Maintenance and repair
3.6 Accessories
3.7 Storage
3.8 Useful life and disposal
14
14
16
16
17
20
21
23
25
26
Index
Foreword
Index

Index
26
27
28
29
29
30
31
32
33
33
33
33
34
35
36
36
6.1 Technical overview
6.2 Handset with locking function
6.3 Locking function for handset
6.4 Operation of the side rails
6.4.1 The non-split side rail
6.4.2 The 2-part aluminium side rails
6.4.3 The 3-part steel side rail
6.5 Operation of the bed extension
6.6 Operating the central brake
6.7 Emergency lowering
6.7.1 Emergency lowering via integrated 9V battery (electric)
6.7.2 Battery change
6.7.3 Emergency lowering of the backrest (manual)
6.7.4 Trendelenburg / Antitrendelenburg function (option)
6.7.5 comfort seating position
6.7.6 lowest position
6.7.7 Staff control panel (accessories) 37
38
39
40
40
41
43
44
45
7. Care, cleaning and disinfection
8. Cause and remedy of malfunctions
9. Maintenance
9.1 Bases
9.2 Maintenance schedule
9.3 Check of first-error safety by means of integrated locking function in the handset
10. Warranty
11. Useful life and disposal
12. Technical specifications 46

4 / 53
46
47
47
47
48
12.1 Technical data (mechanical)
12.3 Technical data Environment
12.4 Classification
12.5 Weights of the individual components
12.6 Identification plates
12.7 Information on electromagnetic compatibility 49
13. Declaration of conformity 52
Index

5 / 53
Foreword
Dear customer,
The team from Harvest Healthcare would like to thank you for the trust you have placed in our Cadence profiling bed
range. With the decision to purchase a care bed from "Harvest Healthcare“ you receive a care product with high
functionality at the highest safety level.
With the purchased nursing bed we can guarantee you optimal lying comfort.
All beds are carefully checked by our staff before delivery.
The care bed delivered to you has left our premises in perfect condition.
When you receive the care bed, the responsibility for its proper and intended operation also passes to you at the
same time.
These instructions for use inform you as the operator and your users in their daily work about the functioning and safe
handling of the care bed.
Please keep the instructions for use at hand near the care bed at all times.
We are convinced that our product will make a positive contribution to your care.
Best regards
Your Harvest healthcare Team
Please read and observe these operating instructions before each use!
If you change ownership, please include these instructions for use.

6 / 53
models
Cadence Comfort with V type
headboard and footboard
Lying surface 90 cm x 200 cm with
wooden side rails
Cadence Comfort with V type
headboard and footboard
Lying surface 90 cm x 200 cm with
2-part split aluminum side rail
Cadence Select
Lying surface 90 cm x 200 cm with
lower headboard and footboard with
2-part split aluminum side rail
Cadence Select
Lying surface 90 cm x 200 cm with
3-part split steel side rail

7 / 53
1. General information
Before the first use:
Read the instructions for use conscientiously and completely!
Please pay particular attention to the various safety instructions. The care bed should be cleaned and disinfected
befo-re first use and before each re-use.
Harvest Healthcare beds carry the CE mark. The healthcare beds meet the requirements for safety and
functionality. The Cadence bed range has been tested according to international standards, which include the
safety requirements for medical devices.
However, these safety requirements can only be met if the user is convinced of the proper condition before using the
care bed (including accessories).
Please note the Medical Device Operator Ordinance (MPBetreibV, 2021).
1.1 Explanation of the symbols used
In these operating instructions, important information is indicated by the following symbols:
Read information with this symbol carefully and observe it urgently. This information is relevant to
safety
This symbol warns of dangerous voltage. There is a danger to life!
This symbol warns of general dangers. There is danger to life and health.
Mark of conformity according to Medical Devices Regulation (EU) 2017/745
manufacturing date
Manufacturer of the medical device
medical device
Serial number
8 / 53
1. Allgemeine Hinweise
Protection of electrical equipment against splashing water
Symbol for device of protection class II, double protective insulation
Symbol for type B application part according to IEC 60601-1
The nursing bed may only be used indoors.
The product must be collected separately in the European Union. Disposal with normal household
waste is not permitted.
Symbol for DC
Symbol for AC
Symbol for safe working load
Symbol for maximum patient weight
Symbol for reading instruction manual
~
1.2 Explanation of the designated groups of persons
Operator
The operator of a medical device is any natural or legal person who is responsible for the operation of the health facility
in which the medical device is operated or used by its employees. Contrary to sentence 1, the operator of a medical
de-vice which is owned by a member of the medical profession or the medical industry and which is brought into a
health facility by this member for use is the relevant member of the medical profession or the medical industry. A
person is also considered to be an operator if he keeps medical devices ready for use outside of health facilities in his
company or facility or in public space. [§2, paragraph 2, MPBetreibV, 2021]
Requirements to be met by the operator
•Please note that for you as the operator of this medical device, the requirements of the Medical Device Operator
Ordinance (MPBetreibV, 2021) are binding.
•The Cadence bed is a medical device and may only be operated and used in accordance with its intended purpose,
the regulations of the MPBetreibV, the relevant legal regulations as well as the generally recognised rules of
technology.
•Only instruct persons to use this medical device who have the necessary training or knowledge and experience and
who have been instructed in the medical device to be used.
•Instruct the user in the proper handling of this medical device and document the instruction in an appropriate form.
IPX4

9 / 53
1. General information
•A combination with other medical devices (including accessories) or with other objects may only be operated and
used if they are suitable for use in this combination, taking into account the intended purpose and the safety of
patients, users, employees or third parties.
User
The user is anyone who uses a medical device on a patient within the scope of the Medical Device Operator
Ordinance (MPBetreibV). [§2, Para. 3, MPBetreibV, 2021]
User requirements
•Use the Cadence bed range only as intended and in accordance with these instructions for use.
•Only use this product if you have been properly instructed in its use and have the necessary training or knowledge
and experience (e.g. nursing staff).
.•Before using the care bed, make sure that it is in good working order and condition
•Observe the Instruction Manual and other safety-related information enclosed.
•If suspected serious events occur in connection with the Cadence bed range, they must be reported to Harvest
Healthcare and the responsible federal authority. Serious incidents occurring in other contracting states of the
Agreement on the European Economic Area must be reported to the competent authorities of this state.
•Suspected Serious events means an event that cannot be ruled out due to an undesirable side effect of a product,
a malfunction, deterioration in the properties or performance of a product, including application errors due to
ergonomic features or an inadequacy of the information provided by the manufacturer is based. Such a suspected
serious event can have led directly or indirectly to death, to a temporary or permanent serious deterioration in the
state of health of a patient, user or other person, as well as to a serious risk to public health
(refer to the Ordinance on the Reporting of Suspected serious incidents with medical devices as well as for the
exchange of information between the responsible authorities - MPAMIV).
Patient / Resident
In these instructions for use, a patient is defined as a person who is in need of nursing care due to his or her illness,
disability or age and is lying in a nursing care bed.
requirements for the patient / resident
It is possible for the patient lying in care bed to independently operate the electrical adjustment functions of the care
bed via the hand switch if he has been instructed in the use of the bed and is mentally and physically able to do so.
Independent use of the Cadence bed by the patient therefore requires that the patient can carry out the adjustment
functions safely and specifically using the hand control and can also free himself from dangerous situations.
Qualified personnel
The operator‘s employees who are authorised to deliver, assemble, dismantle and transport the care bed on the
basis of their training or instruction are referred to as qualified personnel. In addition, these persons are instructed in
the instructions for cleaning and disinfecting the care bed.

10 / 53
2.1 Intended use (application environment)
The Cadence profiling beds are designed for the accommodation of adults with a body height from 150cm and a
body weight from 40kg to max. 200kg. They are suitable for use in senior residences, nursing homes and in home
care - i.e. in appli-cation environments 3 and 4 - and may only be operated under the operating conditions described
in these operating instructions.
The Cadence profiling beds are designed to alleviate or compensate for disability or incapacity and to facilitate
working conditions for the caregiver. Any other use is considered improper and is excluded from possible liability.
Attention: The Cadence profiling beds are not designed for use in hospitals. They are not EX-protected and must not
be operated in hazardous areas.
The Cadence profiling beds may only be used in dry interior rooms. They are only suitable for transporting patients
within the patient‘s room and with the lying surface adjusted to the lowest horizontal position.
The Cadence profiling beds care beds has no connection option for equipotential bonding.
You must therefore take this into account when combining the Cadence bed with other electrical medical devices or
with other mains-operated products.
The operator, as a competent person, must check whether the corresponding combination of the Cadence bed with
other electrical devices is safe during the service life and no unacceptable risks can occur.
The operator of the medical devices is responsible for ensuring that the combination of the devices meets the require-
ments of IEC 60601-1.
Non-electrical medical devices must comply with the IEC or ISO safety standards applicable to these devices if they
are to be used / combined with the care bed.
If cables from other devices are routed in the Cadence bed, precautions must be taken to prevent these cables from
being crushed between parts of the care bed.
Take into account the information and safety instructions in the instructions for use of the electrical devices that you
want to combine with the Cadence bed range (e.g. anti-decubitus alternating pressure systems, feeding systems,
infusion pumps, lamps, etc.) as well as the requirements of the IEC 60601-1 standard (in the current Version).
In this case, all Cadence bed functions must be deactivated for safety reasons for the duration of use via the
integrated locking device on the hand control.
2.2 Unauthorised use
All uses deviating from the intended use, which can then also lead to hazards.
These include, for example:
•Loading of the nursing care bed beyond the permissible safe working load (see para. 12.1 and type plate on
bed frame)
•Operation of the care bed by the patient or occupant who has not received any instruction.
•Use of the nursing bed for children
•Try to move the nursing bed in the braked position
•Use of the nursing bed on a non-horizontal surface (max. inclination 5°)
2. Intended purpose

11 / 53
3.1 General safety instructions
Possible potential dangers which may occur despite proper operation must be pointed out separately during the inst-
ruction. Before initial operation, the user/care personnel must read the operating instructions carefully and in detail.
No objects or body parts of persons may be in the movement area of the care bed while the adjustment functions
are being actuated. Risk of crushing!
Ensure that the care bed cannot be operated by children playing and that there are no pets under the care bed when
the bed is adjusted.
If the psychological or mental condition of the patient requires it, the hand control must be locked via the lock switch
on the back of the hand control (nurse key). The locking function is described in detail in par. 6.3. For this patient
group it may also be necessary to place the hand control outside the patient‘s access area in order to avoid the
danger of strangulation by cables.
Bed adjustments may only be carried out by instructed persons or in the presence of an instructed person.
If a possibly necessary side guard (side rail) is used, pay particular attention to the following instructions:
•Only use side rails approved by Harvet Healthcare as optional accessories. The permissible dimensions can be
found in chapter 12.1.
•The use of incompatible side rails is not permitted and can lead to hazards, e.g. due to trapping.
•The distance between two side rails lying one above the other or between the lower edge of the lower side rail and
the lying surface must not exceed 12 cm.
•Only instructed personnel may operate the side rails.
•Side rails may only be fully raised and locked or fully lowered.
•When lowering the side rails, take care not to drop them.
•No parts of the patient‘s body may protrude over the lying surface or touch the side rails while
the adjustment function is being actuated.
•The side rails only offer protection against rolling out when the backrest and knee adjustment are in the horizontal
position.
•Under no circumstances should side rails be used improperly (e.g. for climbing over or supporting).
•The distance between the top edge of the side rail and the top of the mattress in non-compressed condition must
be at least 22 cm. If the distance is less than the specified minimum, use a side rail elevation.
•When in use, the side rails must not remain in a diagonal position.
Before moving the Cadence bed, disconnect the mains plug from the socket and ensure that the mains plug does
not rub against the floor while moving it.
The mains plug should always be accessible so that in an emergency the device can be disconnected from the mains
supply by pulling it out of the socket.
The mains cable must be exposed and must not be trapped, as it is carried with the height adjustment of the care
bed. Otherwise the mains cable may be torn out of its strain relief and damaged. In addition, the mains plug can be
torn out of the socket and expose electrical cores.
Cables from other devices used in the Cadence bed must not be pinched, crushed or pulled by the functions of the
bed. Take appropriate precautions.
3. Safety instructions

12 / 53
If the mains supply cable or the mains plug is damaged, the complete supply cable with plug must be replaced. The
work may only be carried out by the manufacturer or authorized specialists.
Do not use multiple sockets to connect the mains plug, as liquids can penetrate through them.
(Fire hazard and electrical shock)
Before cleaning and disinfecting the care bed, the mains plug must be disconnected from the mains and securely
hung up. The plugs for the handset and the motors which are plugged into the control unit on the lying surface drive
must be plugged in. This is necessary so that no water can penetrate the control unit.
The maximum duty cycle and safe working load must not be exceeded, otherwise safe operation is no longer guaran-
teed (see technical data).
The Cadence beds must not be used in rooms where there is a risk of explosion.
The care bed may only be dismantled if there is no patient or occupant in it.
3.2 Safety instructions for the operator
Use these operating instructions to instruct each user on safe operation before initial use.
Inform the user of any hazards that may exist if the device is not handled properly.
Only instructed persons may operate the care bed. This also applies to persons who only operate the bed as
representatives.
According to the Medical Devices Regulation (EU) 2017/745 and to the Medical Device Directive 93/42/EEC, care
beds are Class I active medical devices.
This results in obligations for you in accordance with the Medical Device Operator Ordinance (MPBetreibV) in order to
ensure the permanently safe operation of this medical device without endangering patients, users and third parties. For
long-term use of the systems, function checks and visible damage must be carried out and documented at least once
a year (see chapter 9.2).
3.3 Safety instructions for the user
Let the operator instruct you in the safe operation of the Cadence bed.
In particular, observe the general safety instructions as described in para. 3.1.
Bed adjustments may only be carried out by instructed persons or in the presence of an instructed person.
Move the lying surface to the lowest position if you leave the nursing bed unattended with the patient. This reduces the
risk of injury to the patient when getting in and out.
If malfunction or damage is suspected, immediately unplug the power cord from the outlet.
Mark the care bed as a "defect“ and take it out of operation. After that, please inform the responsible operator
immediately.
3. Safety instructions

13 / 53
3.4 Cleaning and disinfection
Before cleaning and disinfection, the mains plug must be disconnected from the mains and securely hung up. The
plug for the handset and the motors, which are plugged into the control at the lying surface drive, must be plugged in.
This is necessary so that no water can penetrate the control unit.
Do not immerse the electrical components in water, but only wipe them off with a damp cloth.
The electrical components must not be sprayed with a high-pressure cleaner or water jet. Only wipe disinfection is
permitted.
To avoid skin irritation, always wear liquid-impermeable gloves during cleaning and disinfection work.
Attention: When spray disinfecting with alcohol-containing agents, there is a risk of explosion and fire when used over
large areas.
3.5 Maintenance and repair
Maintenance measures (inspection and maintenance) and maintenance (repair) may only be carried out by persons
who have at least read the safety regulations, followed these operating instructions and are qualified in accordance
with MPBetreibV (2021) §5.
Maintenance, inspection and repair work are not allowed to be carried out on the care bed when it is in use and the
patient is in it.
In order to detect possible defects in time and to ensure safe use, a technical check (visual and functional check) must
be carried out by qualified personnel at least once a year according to the maintenance schedule (see chapter 9.2)
after a longer period of inactivity and before each reuse.
If the tests reveal errors, damage or defects, the care bed may no longer be operated. Maintenance of the Cadence
bed must be carried out by qualified personnel in accordance with MPBetreibV (2021) §5.
Only original spare parts and accessories of the manufacturer may be used, otherwise all warranty and product
respon-sibility are excluded
The 9V block battery is the energy storage device for electrical emergency lowering in the event of a power failure. The
energy storage is sufficient for max. one emergency lowering and must then be replaced. If the expiry date of the
batteries has exceeded, they must also be replaced immediately. As batteries are self-discharging, it is recommended
to replace them every two years if they are not used. Make sure that this is an alkaline manganese battery of type
6LR61 and that only this type may be used. Empty batteries must be disposed of in an environmentally friendly
manner.
3. Safety instructions

14 / 53
3.6 Accessories
An erector is supplied as an accessory whose safe working load of 80 kg must not be exceeded. The trapeze bar is
not used to lift persons, but makes it easier to change from a lying position to a sitting position or to change the
position. The trapeze bar must not be swivelled outside the care bed and must only be used within its permissible
adjustment range, which is defined by the tube holder on the bed. Otherwise the care bed may tip over completely
and lead to serious injuries.
Please only use mattresses that are compatible with the side rails supplied. The distance between the mattress
surface in the non-compressed state and the upper edge of the upper side rail must be at least 22 cm. If the distance
is less than this, a side guard must be used. As a rule, a mattress thickness of 12 cm is suitable.
Make sure that the dimensions of the mattress match the dimensions of the lying surface of your Cadence bed. When
using mattresses that are not compatible with this bed, hazards can arise, e.g. through falling out, trapping, etc.
Another accessory for the Cadence beds is a care bed extension that can be retrofitted and offers the option of
increasing the bed length to 220cm or 230cm. Note the descriptions in Chapter 5.2.
The following accessories are also possible for the Cadence ed range:
•Side rail pad
•reading light
•Underbed lighting with motion detector
•Pluggable tablet
•wall deflection roller
•CPR
3.7 Storage
If the Cadence bed is to be stored for a longer period of time, the 9V block battery should be removed as a
precaution to prevent damage to the care bed from any leaking liquid.
3.8 Useful life and disposal
With correct operation and appropriate use, this care bed has an expected service life of 7 to 10 years.
The care bed must not be disposed of with normal household waste at the end of its service life. For environmentally
friendly disposal, please contact your local authority or Harvest Healthcare.
The electrical components (power supply units, control units, drives and hand controls) of these beds are to be
treated like electronic waste in accordance with WEEE Directive 2012/19/EU (Waste Electrical and Electronic
Equipment) and disposed of properly.
The components used conform to the directive 2011/65/EU (RoHS II) on the restriction of the use of certain
hazardous substances in electrical and electronic equipment.
When disposing of it, please note that the care bed or its accessories can be contaminated and contaminated with
germs. Damage can also result in sharp edges, splintering, etc. These can lead to health risks.
3. Safety instructions

15 / 53
4. Control of delivery and scope of delivery
On receipt of the delivery and before commissioning, check whether the nursing bed is damaged. Complain visible
damage immediately to the delivering company.
After unpacking, please check that the delivery is complete. You will receive a fully assembled Cadence bed
consisting of the following parts:
a. wooden headend
b. wooden footend
c. Electrically adjustable backrest
d. Electrically adjustable thigh support
e. Mechanically adjustable lower leg support
f. Hand switch with nurse key
g. Staff control panel (accessories)
h. Mechanical grid fitting for adjusting the lower leg
support
i. Electric drive for backrest with slide-on power supply
unit
j. Electric drive for thigh support
k. Mains cable with SMPS
l. Mattress guide
m. Wooden side rails (4 pieces)
n. Release button for side rail locking
o.Side rail guide
p. Mechanically adjustable castors
q. Central brake
r. Instruction for use
a
p
l
f
h
k
o
n
c
b
d
eg
i
j
m
q
r

16 / 53
5. Assembly and commissioning
a
a
b
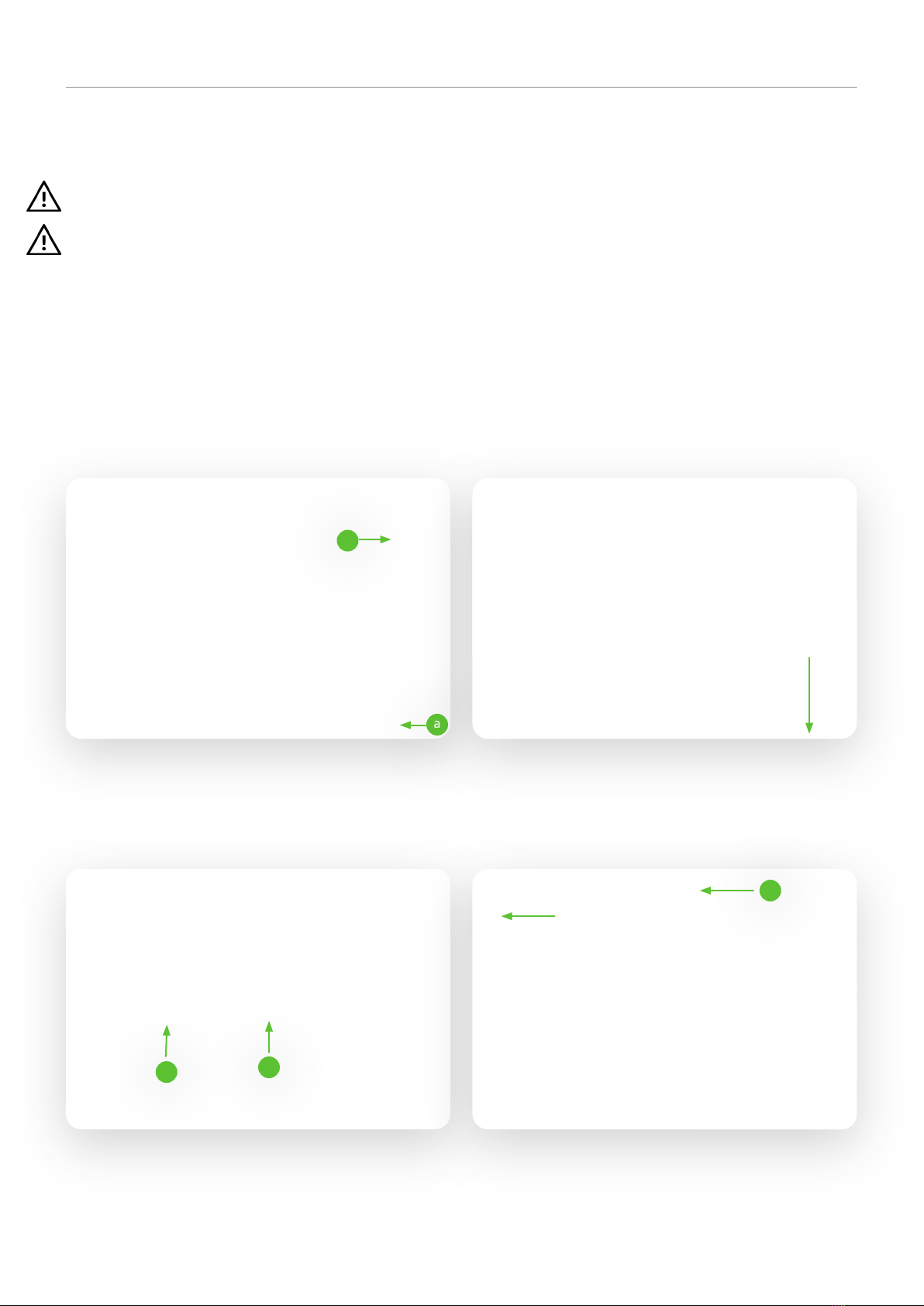
Slide the fixed loop of the triangle belt over the first bolt of the erector (a) and check that it is securely held by pulling
the triangle handle downwards. Fix the loop of the triangle belt only between these two bolts.
The length of the strap of the triangle handle can be adjusted by the buckle. Select an adjustment that allows the user
to easily reach the handle when lying down (usually between 55-70 cm measured from the upper edge of the mattress).
Make sure that the belt is securely fastened again.
Note: The Cadence bed range is supplied fully assembled.
5.1 Assembly of Erector with triangle handle (accessory)
With the help of the erector, the patient can stand up and move more easily into another position. A triangle handle is
attached to the erector.
Mount the erecting yoke by putting the two parts together (a) and screwing the star grip screw into the threaded
hole(b) and tightening it! Insert it into the erecting fixture in the lying surface.
Make sure that the locking cylinder pin (c) engages in the recess of the erecting fixture.
Attention: The erecting bracket must not be used outside the latching mechanism.
c

17 / 53
5. Assembly and commissioning
ab
a. Production month
b. Production year
The triangle handle has a shelf life of at least 5 years under normal use (see embossing of production date). It is then
recommended to replace the triangle handle.
5.2 Mounting the bed extension (Accessories)
The Cadence beds offers the possibility of extending the lying surface up to 220 cm by means of the
integrated care bed extension, in order to be able to provide even larger patients with optimal lying comfort while
maintaining the same functionality.
Note: If you want to extend the to 220cm, you will need the following additional components to keep the care bed
in proper condition. These components must be purchased in advance.
When using continious wooden side rails
No. Component Article number Unit
1bed extension
(bed extension, Pull catch/cable, fastening material) BC 9.04.0655340 Set
2Extended side rails (4 side rail bars) if undivided side
rails were previously used 276 piece
3 Side rail end caps BC 4.03.1990000 Set
420cm or 30cm mattress extension (1 piece) or
one mattress 90x220/230cm piece
5If visually desired, extended side panels
(2 pieces) for the bed extension
HO-SBL-01-PFR5320
-PFR5320 = beech Set
When using split side rails
No. Component Article number Unit
1bed extension
(bed extension, Pull catch/cable, fastening material BC 9.04.0655340 Set
220cm or 30cm mattress extension (1 piece) or
one mattress 90x220/230cm piece
3Protector BC 9.04.0653380 piece
18 / 53
5. Assembly and commissioning
a
b
Consider the following assembly instructions for the use of the bed extension:
The bed extension must not be used as a seat!
The patient must not be in the h bed while the bed extender is being set up.
1. Remove the mattress from the care bed.
2. Dismantle the side rails. To do this, unscrew the socket screws (a) at the bottom of the foot section and pull the
support block out of the side rail. For this dismantling step, the side rail bars should first be pulled up and locked in
place.
3. Press the side rail lock release button (b) on one side and carefully lower the side rails until they have slid comple-
tely out of the guide rails. Keep the side rail stiles for possible dismantling.
4. Loosen the two hexagon socket screws (c) underneath the lying surface frame on the foot side.
5. Pull the foot section out of the lying surface frame (d).
cc
d

19 / 53
5. Assembly and commissioning
6. Dismantle the existing brackets (e) on the foot section plate.
7. Place the care bed extension (f) on the drill holes (g) of the previously dismantled angles of the foot section and
screw them tight with the 4 screws.
e
f
8. Insert the foot section incl. the care bed extension into the openings of the lying surface frame (h). The hooks (i) of the
bed extension must engage in the last cross strut of the lying surface frame.
g
g
h
i
Table of contents
Other Prism Healthcare Medical Equipment manuals
Popular Medical Equipment manuals by other brands
Intersurgical
Intersurgical i-view Instructions for use
Datex-Ohmeda
Datex-Ohmeda Aestiva/5 Operation manual

Med-Mizer
Med-Mizer ActiveCare-Deluxe user manual

meridius
meridius Far Infrared Heating Mat manual

Orthopedic Systems
Orthopedic Systems Jackson Spinal Table System user guide
Sirona
Sirona CEREC AC BLUECAM operating instructions