Proteus SPC-0800 Operation manual

LBL‐0176,Rev1|UserSupplementalInformation,
Proteus®Patch
Page1of1Effective:19DEC2013
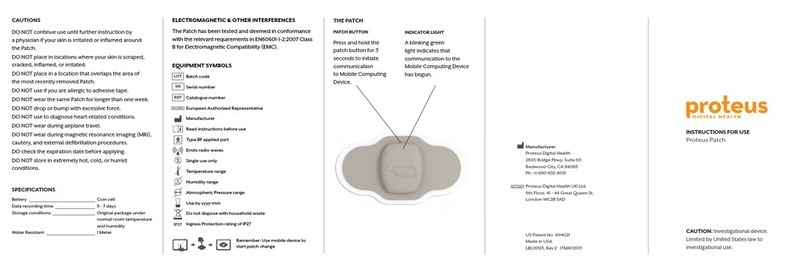
THEPROTEUSPATCH
SPC‐0800
USERSUPPLEMENTALINFORMATION
TableofContents
1.TECHNICALINFORMATION................................................................................................................2
1.1–Classification....................................................................................................................................2
1.2–EnvironmentalConditions..........................................................................................................2
1.3–MinimizingSkinIrritation............................................................................................................2
1.4–ProtectionagainstIngressofSolidsandLiquids.................................................................2
1.5–AvoidingUnsafeUseConditions..............................................................................................2
1.6–InformationonElectromagneticandOtherInterferences...............................................3
1.7–InformationontheRadioSubsystem......................................................................................3
1.8–EuropeanR&TTEDeclarationofConformity.........................................................................7
1.9–CISPRInterferenceStatement....................................................................................................7
1.10–FCCInterferenceStatement.....................................................................................................8
1.11–FCCWirelessNotice.....................................................................................................................8
1.12–FCCIdentifier.................................................................................................................................8
2–DISPOSALOFWASTEPRODUCTS..................................................................................................8
3–MANUFACTURERCONTACTINFORMATION.............................................................................9
LBL‐0176,Rev1|UserSupplementalInformation,
Proteus®Patch
Page2of2Effective:19DEC2013
1.TECHNICALINFORMATION
1.1–Classification
Caution:Federal(U.S.A)lawrestrictsthisdevicetosalebyorontheorderofaphysician.
TheProteusPatchiscategorizedasClassII(inUS)andIIa(inEU).
TheUser(patient)istheintendedOperatoroftheProteusPatch.
1.2–EnvironmentalConditions
TheProteus®Patchisintendedforstorageandoperationinaroom‐temperatureenvironment.
ConditionTemperatureHumidityPressure(Altitude)
Operating20C–28C 15%‐93%700hPa–1060hPa
Storage20C–28C15%‐93%700hPa–1060hPa
Transport2C–38C15%‐85%700hPa–1060hPa
1.3–MinimizingSkinIrritation
TheProteusPatchhasbeendesignedtominimizethepossibilityofskinirritation.Observingthese
cautionswillreducethelikelihoodofskinirritationorbruisingunderthePatch:
DONOTcontinueuseuntilfurtherinstructionbyaphysicianifyourskinisirritatedor
inflamedaroundthepatch.
DONOTplaceinlocationswhereyourskinisscraped,cracked,inflamed,orirritated.
DONOTplaceinalocationthatoverlapstheareaofthemostrecentlyremovedPatch.
DONOTuseifyouareallergictoadhesivetape.
DONOTwearthesamePatchformorethanoneweek.
DONOTdroporbumpwithexcessiveforce.
1.4–ProtectionagainstIngressofSolidsandLiquids
TheProteusPatchhasanIngressProtectionratingofIP27.Thismeansthattheenclosurehasno
penetrationsandithasbeenratedforimmersioninliquidupto1mdepth.Forcontinuedsafety,
shouldtheenclosurebecomepenetratedortorn,removethePatchimmediatelyandreplaceitwith
anewone.
1.5–AvoidingUnsafeUseConditions
TheProteusPatchisnotadiagnosticdevice.DONOTattempttouseittodiagnoseheart‐related
conditions,anincorrectdiagnosismayresult.

LBL‐0176,Rev1|UserSupplementalInformation,
Proteus®Patch
Page3of3Effective:19DEC2013
TheProteusPatchhasnotbeentestedorapprovedassafeforoperationduringairtravel.DONOT
usethePatchduringairtravel;itmayinterferewiththeaircraftnavigationalinstruments.
TheProteusPatchhasnotbeentestedorapprovedforuseinthepresenceofstrongmagneticor
electricfields.DONOTwearthePatchduringmagneticresonanceimaging(MRI),cautery,and
externaldefibrillationprocedures.DamagetothePatch,yourskin,oranunexpectedmagnetic
attractionmayresult.PleaseinformyourhealthcareprofessionalthatthePatchmustberemoved
priortoengaginginoneoftheseprocedures.
WARNING:Nomodificationofthisequipmentisallowed.ModifyingtheProteusPatchmaycausea
safetyhazardfortheuser.
1.6–InformationonElectromagneticandOtherInterferences
TheProteusPatchhasbeenevaluatedanddeemedcompliantwiththerequirementsinEN60601‐1‐
2ClassBforElectromagneticCompatibility(EMC).MedicalElectricalEquipmentneedsspecial
precautionsregardingEMCandneedstobeinstalledandputintoserviceaccordingtotheEMC
informationprovidedinthisUserManual.PortableandmobileRFcommunicationsequipmentcan
affectMedicalElectricalEquipment.TheProteusPatchshouldnotbeusedadjacenttoorstacked
withotherelectromagneticequipment.Ifadjacentorstackedusewithotherelectromagnetic
equipmentisnecessary,verifythattheProteusPatchoperationisnormalintheconfiguration(s)in
whichitwillbeused.
1.7–InformationontheRadioSubsystem
TheProteusPatchincorporatesaBluetoothTmradiosubsystemwhichiscompliantwiththe
Bluetoothstandard.ThefollowinginformationisprovidedtosatisfytherequirementsofEN/IEC
60601‐1‐2:
TheBluetoothradiotransmitsandreceiveson40frequencybandswhichareequallyspacedat
2MHzintervalsbetween2402MHzand2480MHz.
Theeffectivereceivebandwidthis1.25MHz.
Thetransmitmodulationisfrequency‐hoppingusingGFSK(GaussianFrequencyShiftKeying)witha
bandwidth‐bitperiodproductBT=0.5.TheModulationindexisbetween0.28and0.35.
Theeffectiveradiatedpoweris‐15dBm(P=0.032mW)
Guidanceandmanufacturer’sdeclaration–electromagneticemissions
TheProteusPatchisintendedforuseintheelectromagneticenvironmentspecifiedbelow.The
customerortheuserofProteusPatchshouldassurethatitisusedinsuchanenvironment.
EmissionstestComplianceElectromagneticenvironment–guidance
RFemissions
CISPR11
Group1
ThePatchusesRFenergyonlyforitsinternalfunction.
Therefore,itsRFemissionsareverylowandarenotlikely

LBL‐0176,Rev1|UserSupplementalInformation,
Proteus®Patch
Page4of4Effective:19DEC2013
tocauseanyinterferenceinnearbyelectronicequipment.
RFemissions
CISPR11
ClassBThePatchissuitableforuseinallestablishments,
includingdomesticestablishmentsandthosedirectly
connectedtothepubliclowvoltagepowersupply
networkthatsuppliesbuildingsusedfordomestic
purposes.
Harmonic
emissions
IEC61000‐3‐2
Notapplicable
Voltage
fluctuations/
flickeremissions
IEC61000‐3‐3
Notapplicable

LBL‐0176,Rev1|UserSupplementalInformation,
Proteus®Patch
Page5of5Effective:19DEC2013
Guidanceandmanufacturer’sdeclaration–electromagneticimmunity
TheProteusPatchisintendedforuseintheelectromagneticenvironmentspecifiedbelow.The
customerortheuseroftheProteusPatchshouldassurethatitisusedinsuchanenvironment.
ImmunitytestIEC60601
testlevel
Compliance
level
Electromagneticenvironment–
guidance
PortableandmobileRF
communicationsequipmentshouldbe
Guidanceandmanufacturer’sdeclaration–electromagneticimmunity
TheProteusPatchisintendedforuseintheelectromagneticenvironmentspecifiedbelow.The
customerortheuserofthePatchshouldassurethatitisusedinsuchanenvironment.
ImmunitytestIEC60601testlevelCompliance
level
Electromagneticenvironment–
guidance
Electrostatic
discharge(ESD)IEC
61000‐4‐2
+/‐6kVcontact
+/‐8kVair
+/‐6kV
contact
+/‐8kVair
Floorsshouldbewood,concrete
orceramictile.Iffloorsare
coveredwithsyntheticmaterial,
therelativehumidityshouldbeat
least30%.
Electricalfast
transient/burst
IEC61000‐4‐4
+/‐2kVforpower
supplylines
+/‐1kVfor
input/output
lines
Notapplicable
SurgeIEC61000‐4‐
5
+/‐1kVline(s)to
line(s)
+/‐2kVline(s)toearth
Notapplicable
Voltagedips,short
interruptionsand
voltagevariations
onpowersupply
inputlines
IEC61000‐4‐11
<5%UT(>95%dipin
UT)
for0,5cycle
40%UT(60%dipinUT)
for5cycles
70%UT(30%dipinUT)
for25cycles
<5%UT(>95%dipin
UT)
for5s
Notapplicable
Powerfrequency
(50/60Hz)
magneticfield
IEC61000‐4‐8
3A/m3A/mPowerfrequencymagneticfields
shouldbeatlevelscharacteristic
ofatypicallocationinatypical
commercialorhospital
environment.
NOTEUTisthea.c.mainsvoltagepriortoapplicationofthetestlevel.
Table of contents
Other Proteus Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual