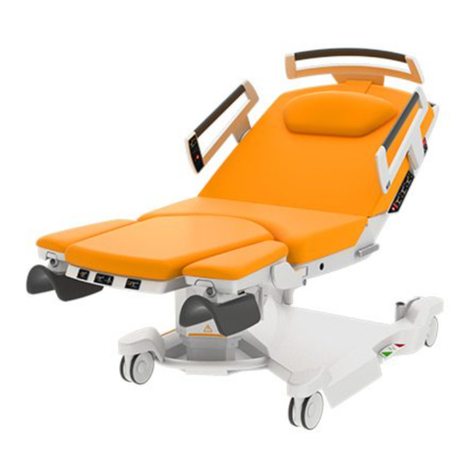
BORCAD Medical AVE PPA-AX30 User manual
BORCAD Medical a.s., CZ - 739 45 Fryčovice 673
T: +420 558 640 631, W: www.borcadmedical.com
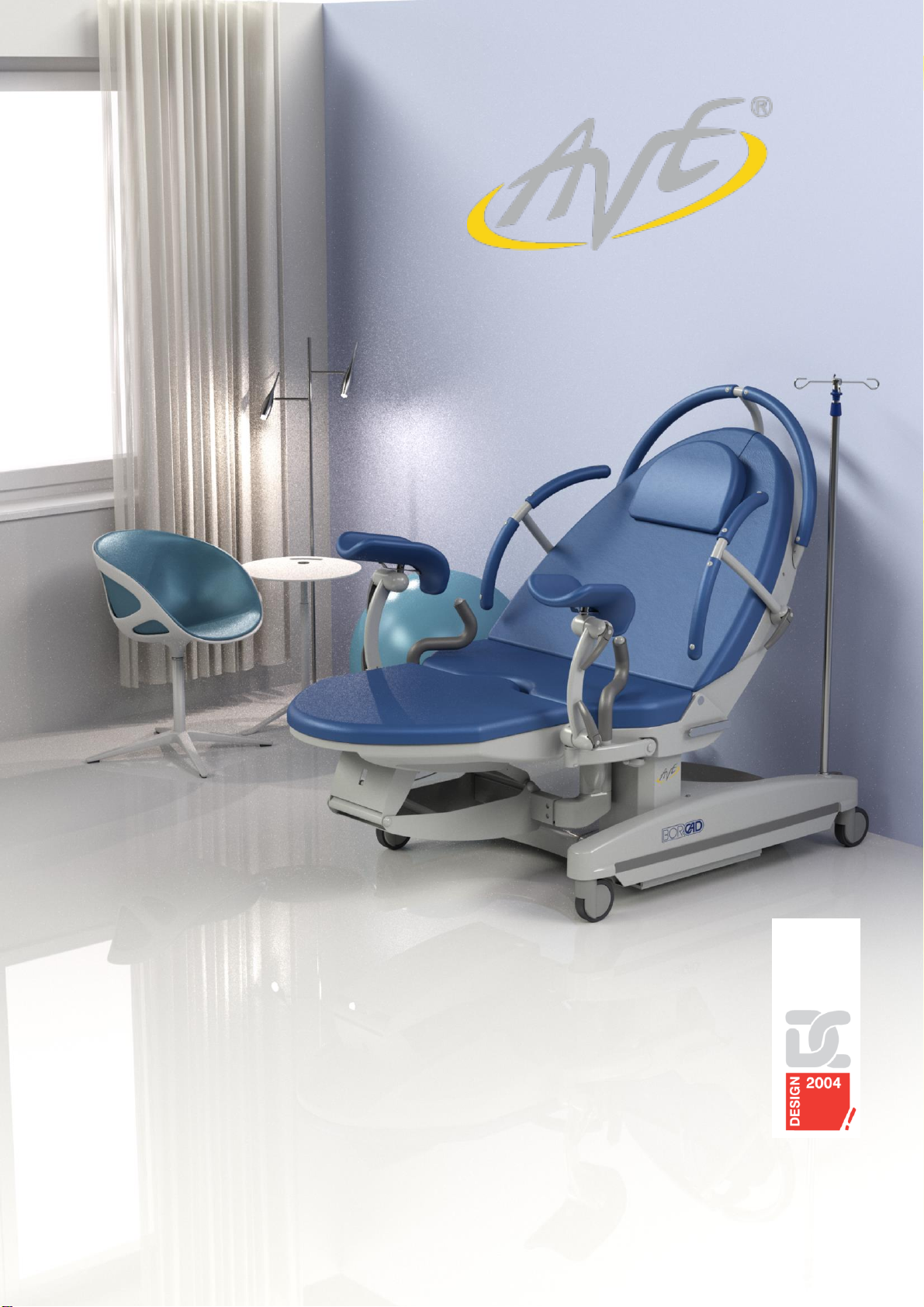
Birthing bed AVE
UN-PACKING MANUAL
INSTRUCTION MANUAL
PPA-20161212-EN

2
BORCAD Medical a.s.
739 45 Fryčovice 673, tel.: +420 558 640 611, fax: +420 558 668 087 www.borcadmedical.com
INTRODUCTION........................................................................................................................... 3
1 UN-PACKING MANUAL............................................................................................................7
2 OVERVIEW OF THE BASIC PARTS OF AVE BIRTHING BED.............................................13
2.1 OVERVIEW OF VERSIONS......................................................................................................14
2.2 SAFETY...............................................................................................................................14
2.3 SETTING THE PRODUCT........................................................................................................20
2.4 OPERATING THE BED............................................................................................................23
2.4.1 Motor setting (Hand controller)..................................................................................23
2.4.2 Motor setting (Foot controller) ...................................................................................24
2.4.3 Motor setting (Wireless foot controller)......................................................................25
2.4.4 Manual setting of the CPR and Trendelenburg.........................................................30
2.4.5 Adjusting the foot section ..........................................................................................31
2.4.6 Adjusting the legrests ................................................................................................33
2.4.7 Separable leg supports (PPA-00.46.x)......................................................................36
2.4.8 Leg holders (joint systém-PPA-00.10-X)...................................................................38
2.4.9 Adjustment of leg support..........................................................................................39
2.4.10 Handle holders for hands for leg rests.....................................................................39
2.4.11 Adjusting the tray.....................................................................................................41
2.4.12 Adjusting the lumbar support (only for version PPA-CX) ........................................41
2.4.13 Turning on the massage segment (only for version PPA-BX).................................42
2.4.14 Equipotential coupling .............................................................................................43
2.4.15 Exchangeability of mattresses / upholstery of birth bed bearing surfaces ..............44
3 ACCESSORIES .......................................................................................................................45
3.1 PPA-29-X SUPPORTING UPHOLSTERED BAR.........................................................................45
3.2 PPA-33 LARGE STAINLESS STEEL BOWL -10 L......................................................................45
3.3 PPA-535 UNDERCARRIAGE LIGHT........................................................................................46
3.4 PPA-36 TELESCOPIC INFUSION STAND.................................................................................46
3.5 PPA-48.21 FOOT CONTROLER.............................................................................................47
3.6 PPA-556 WIRELESS FOOTCONTROLER ................................................................................47
3.7 PPA-65 PLASTIC BASKET FOR ACCESSORIES........................................................................48
3.8 PPA-083 BOWL FOR INSTRUMENTS......................................................................................48
3.9 PPA-70-X HEAD REST SEMICIRCULAR-ADJUSTABLE..............................................................49
3.10 PPA-71 HOLDER OF REMOTE CONTROL..............................................................................49
3.11 ZK-05.X DOCTOR´S CHAIR ................................................................................................49
3.12 GKB-076 DOCTOR´S CHAIR (ERGONOMIC) .........................................................................49
3.13 PPA-00.37 SIDE SUPPORT WITHOUT UPHOLSTERY (PAIR)...................................................50
3.14 PPA-00.35-X SIDE SUPPORT WITH UPHOLSTERY (PAIR)......................................................50
3.15 PPA-00.36-X DOUBLE SIDE SUPPORT WITH UPHOLSTERY (PAIR).........................................50
4 CARE .......................................................................................................................................52
4.1 CLEANING AND DISINFECTION...............................................................................................52
5 MAINTENANCE.......................................................................................................................55
5.1 LIST OF ERROR MESSAGES AND OTHER GENERATED MESSAGES.............................................55
5.2 TECHNICAL SPECIFICATION...................................................................................................57
5.3 ELECTRIC PARAMETERS.......................................................................................................58
5.4 ATTACHED PARTS OF PRODUCT............................................................................................59
5.5 TRANSPORTATION AND STORAGE .........................................................................................60
5.6 AMBIENT CONDITIONS FOR OPERATION .................................................................................60
5.7 SERVICE REPAIRS................................................................................................................61
5.8 ENVIRONMENT PROTECTION ................................................................................................63
3
BORCAD Medical a.s.
739 45 Fryčovice 673, tel.: +420 558 640 611, fax: +420 558 668 087 www.borcadmedical.com
Introduction
Please read this complete instruction manual carefully which is designed to
familiarize you with the correct use and the parameters of your new birthing
bed. Please always follow the instructions contained in this manual and use the
bed always only in accordance with these instructions.
Please keep these instructions always near the bed throughout its operation.
Every person operating the birthing bed must read and understand the contents
of these instructions.
Very important information is marked throughout the manual by the following
symbols:
Logo
Meaning
EU legislation compliant product
Quality management systems certification authority marking
Quality management systems certification authority marking
The upholstery meets the requirements on resistance to ignition
according to BS 7176 in the Medium hazard category.
The upholstery meets the requirements of BS EN 1021-1, BS
EN 1021-2, BS 5852 –resistance to ignition sources 5
4
BORCAD Medical a.s.
739 45 Fryčovice 673, tel.: +420 558 640 611, fax: +420 558 668 087 www.borcadmedical.com
Pictogram
Meaning
Warning
Warning:
Dangerous electric voltage
This symbol introduces any information, that may help you to
avoid operative problems
B type accessory part
Serial number
REF
Type number explanation
int x/y
Marking of intermittent operation, i.e. if the product has been
continuously operated for time period “x”, then it must rest idle
for time period “y”. For example int 10 / 20 means that after 10
minutes of continuous use /positioning the product must be out
of use/not positioned for 20 minutes
IP
Design protection against the ingress of water, hazardous
contact and the ingress of foreign objects according to IEC 529
Safe operational load
Safe patient load
Product weight
Battery marking
Producer
Date of production
5
BORCAD Medical a.s.
739 45 Fryčovice 673, tel.: +420 558 640 611, fax: +420 558 668 087 www.borcadmedical.com
Follow the instructions manual
Equipotentiality
Packaging material:
“Keep dry”
Packaging material:
“Fragile!“
Packaging material:
“This side up”
Temperature limitation
Transportation marking:
„Relative air humidity“.
Transportation marking:
“Air pressure”.
Marking according to the directive EC 2002/96/EC (Directive
about the disposal of old electric and electronic appliances).
Symbol for: “Do not dispose of the product into communal
waste. Use special collection points for old electronic
appliances.“
The medical device includes an RF transmitter which emits non-
ionizing electromagnetic radiation.
Cardiopulmonary resuscitation

6
BORCAD Medical a.s.
739 45 Fryčovice 673, tel.: +420 558 640 611, fax: +420 558 668 087 www.borcadmedical.com
Abbreviation
Meaning
LED
Light emitting diodes
VA
Power intake units
VGA
Computer standard for computer imaging technology
USB
Universal serial bus
LCD
Liquid crystal display
IT
Information technology
PC
Personal computer
dB
Sound intensity unit
hPa
Pressure unit
ČSN
Protected marking of the Czech technical norms
Hz
Frequency unit in the SI system
EMC
Electromagnetic compatibility
VF
High frequency
ME
Medical equipment
CISPR
International Special Committee on Radio Interference
Any potential queries address please to the authorized representative or directly
to the producer BORCAD Medical a.s.

7
BORCAD Medical a.s.
739 45 Fryčovice 673, tel.: +420 558 640 611, fax: +420 558 668 087 www.borcadmedical.com
1 Un-packing manual
Carefully cut through prospect and manual pockets and remove them out.
Carefully cut through cover following bottom edge of palette.

8
BORCAD Medical a.s.
739 45 Fryčovice 673, tel.: +420 558 640 611, fax: +420 558 668 087 www.borcadmedical.com
Carefully cut through foil witch hold electrical cable and put into electricity.
Cut through the tape around the box and remove the trapeze.

9
BORCAD Medical a.s.
739 45 Fryčovice 673, tel.: +420 558 640 611, fax: +420 558 668 087 www.borcadmedical.com
Raise the bed back into the position 60 °, the driver is attached to the drawbar
engine back under the bed.
Cut the tape on the tray and gradually remove all the accessories.

10
BORCAD Medical a.s.
739 45 Fryčovice 673, tel.: +420 558 640 611, fax: +420 558 668 087 www.borcadmedical.com
Unscrew the medical chair if included.
Remove the box with a bowl if it is included.
All wheels rotate in the direction to the longer end of the palette and lock wheels
in the direction.

11
BORCAD Medical a.s.
739 45 Fryčovice 673, tel.: +420 558 640 611, fax: +420 558 668 087 www.borcadmedical.com
Gradually unscrew the screw holding the bed. If is possible try them get down
on the floor.
Put polystyrene runs to the edge of the palette. Gently push down on the bed in
the direction of the arrows. Go down from the palette.

12
BORCAD Medical a.s.
739 45 Fryčovice 673, tel.: +420 558 640 611, fax: +420 558 668 087 www.borcadmedical.com
Connecting the backup batteries
1. Lift high plastic cover (A) and remove low plastic cover (B).
2. There are electronical components under the plastic cover. Plug in
connector C into position D (marked as „BAT“) in controlbox unit.
3. To plug in the connector into position D in controlbox, it is necessary to lift
the plastic cover of connectors. It is necessary to put the plastic cover into
original position after the connector is plugged in.

13
BORCAD Medical a.s.
739 45 Fryčovice 673, tel.: +420 558 640 611, fax: +420 558 668 087 www.borcadmedical.com
2 Overview of the basic parts of AVE birthing bed
1. bed part, for version BX with massaging segment, for version CX with
lumbar segment
2. seat section
3. foot section
4. castor, directional 1x
5. castor with brake 3x
6. plastic covers of chassis
7. leg rest with new system of joint control
8. headrest adjustable by sliding on the back
9. handle of back part
10.eurolath along seat section
11.tray on swinging holder
12.control lever for positioning of foot section in horizontal position
13.security screw of holder of leg rest
14.protective ledge of the plastic cover of chassis
15.control light for power supply
16.holder for infusion stand
6
14
9
10
16
5
15
4
8
12
13
3
2
11
1
7

14
BORCAD Medical a.s.
739 45 Fryčovice 673, tel.: +420 558 640 611, fax: +420 558 668 087 www.borcadmedical.com
2.1 Overview of versions
PPA-AX30
basic type
PPA-BX30
type with massaging segment
PPA-CX30
type with lumbar segment
PPA-AX36
basic type, upholstery with zipper
Color versions of cushioned parts (symbol X):
Type
Color (X):
P
orange
M
corn yellow
F
ocean green
B
brilliant blue
T
pink
D3
Pastel orange
The number “36” means special upholstery on request.
2.2 Safety
Standards
The product meets the requirements of valid standards EN 60 601-1 and EN
60 601-1-2
According to the Directive on Medical instrumentation 93/42/EEC the birthing
bed is classified as medical instrument of 1st class.
Function
The essential functions are lying, sitting and supporting the woman in
labour. Additionally, it is the CPR position.
Delivery bed is used by mothers before and during delivery. A bed equipped
with adequate accessories offers birth mothers the opportunity to take up their
optimum position for birth. Delivery bed can be used together with a set of
cushions.Before use it is necessary to disinfect the bed with common
disinfectant solutions, place a hygienic cover over its washable surface using
typical draping. Positioning and handling the bed is not an obstacle in providing
health care. Handling the foothold part, especially by retracting it (fast way to
store it under the seat/back part) at the end of the second stage of delivery it is
possible to create more space to perform obstetrical and vaginal surgeries and
it is also useful for practical training of medical students and midwives.

15
BORCAD Medical a.s.
739 45 Fryčovice 673, tel.: +420 558 640 611, fax: +420 558 668 087 www.borcadmedical.com
Delivery bed provides the possibility to perform an acute Caesarean section
either under general anaesthesia or NAB anaesthesia (neuraxial block). It also
enables for immediate preparation of the birth mother for a Caesarean section
and her positioning on the side until the foetus is removed (supine syndrome
prevention), also the use of other medical devices (resulting in ME system) for
continuous control of reduced venous return, blood pressure, routine ECG
monitoring, pulse oximeter, NIBP, and capnometry. During procedures it is
possible to use approved surgical devices (VF) for making incisions or
coagulation. If it is necessary to perform cardiopulmonary resuscitation, it can
be performed on this delivery bed including the use of the defibrillator. Delivery
bed is also equipped with a Trendelenburg position, which can be operated by
both electric drive and mechanically –manually. After delivery and the transfer
of a birth mother it is necessary to wash and disinfect all the easily removable
parts and light upholstery together with plastic parts and covers again.
The upper handle on the backrest can, in some cases (given the
customs of a particular school or workplace) provide slightly worse
access for the anaesthesiologist to the patient.
For a Caesarean section it is necessary to remove the trapeze bar from
the delivery bed and lower the foot section to the lowest position.
Indication
Delivery bed is designated to be used by mothers ready for spontaneous
vaginal delivery and also as aid to search for individual positions providing relief
in all delivery stages.
Based on the supplied equipment it is possible to conduct the delivery in a
typical or an alternative way –positions: lying on the back, the semi-recumbent
position, on all four limbs, lying on the side, in a squatting position, in a
suspended position or squatting down with the help of a partner. In case of
acute, urgent or emergency needs it is possible to perform a Caesarean
section.
Contra-indications
A relative contra-indication is the use of the delivery bed for elective Caesarean
sections (planned) when it is planned to perform the surgery in a standard
operation room with adequate equipment.
Interaction
When using the delivery bed as part of the medical system, certain elements
(medical devices) may interact. Before this application it is necessary to follow
recommendations provided in the user manual of the delivery bed.
16
BORCAD Medical a.s.
739 45 Fryčovice 673, tel.: +420 558 640 611, fax: +420 558 668 087 www.borcadmedical.com
General instructions
The birthing bed may only be operated where the conditions of valid standards
for el. distribution in medical facilities are met. According to the Act on Means of
Medical Instrumentation the product may only be operated by persons with
adequate qualification.
Performance of servicing works: BORCAD Medical a.s. authorized service may
only responsibly evaluate the safety technological features if the operators,
repairs, alternations, etc., had been performed only at the producer authorized
locations and the product is operated in full conformity with the instruction
manual. Birthing bed should not be used if there are obvious mechanical
defects of upholstery, covers and other supporting parts.
General safety:
Never use other than original accessories
See to correct fixation and setting of accessories (tightening of handles etc.)
Do not dismantle and/or interfere with motors, control unit, etc.
The AVE bed must not be used in medical facilities with risk of fire or
explosion caused by air or in combination with nitrogen oxide and
anesthetics or cleaning agents.
Structural alternations reserved!
The bed must be equipotentially connected with other medical devices to
prevent fire in combination with flammable substances and anesthetics
during minor surgical procedures using high-frequency (HF) instruments.
Never use other than original accessories. All original accessories are
marked with original label containing the name and brand of the
manufacturer, designation of the product and a code containing further
information (serial number or batch ID and date of manufacture).
17
BORCAD Medical a.s.
739 45 Fryčovice 673, tel.: +420 558 640 611, fax: +420 558 668 087 www.borcadmedical.com
Electrical safety and EMC:
Since the unit is powered from the network, it may cause interference
with sensitive equipment due to generation of electromagnetic field. In
order to reduce the influence of undesired electromagnetic effects, the
bed was designed in compliance with ČSN EN 60601-1-2. To prevent
the occurrence of these problems the bed must be used in compliance
with this manual.
If there is a fluctuation in the voltage range (+/- 20 V) then it is
necessary to connect the product to voltage regulator. Otherwise the
electronics can be damaged.
To avoid risk of electric shock, this chair must be connected to power
main with Protective earthing
The chair must be positioned in such way, so if its necessary could be
immediately disconnected from the power main
Modifications to this chair is forbidden
Before plugging check the power cable for mechanical damage.
Before plugging in check the voltage of the network for conformity with
information on the type label of the bed. A plugged-in bed is indicated by a
green control light on the foot of the bed.
Power cable of the bed must not be connected through an extension cord
but directly into the power socket.
Avoid hanging the power cable over moving parts of the bed. When
accumulators are in use, the cable may be damaged!
No input cable of this or other devices can lead over the bed and /or over
the patient.
Within the space of 1.5 m, within the patient space, only medical equipment
meeting standard EN 60601-1 or medical equipment with certification
according to IEC may be used.
Birthing bed is not intended for use in areas enriched with oxygen
(concentration higher than 25% or partial pressure reaching 27.5 kPa).
The birthing bed is intended only for use by medical professionals.
The birthing bed may cause radio interference, which can have effects
on the operation of nearby equipment. It may be necessary to take
measures to reduce these effects, such as redirection, relocation or
shielding of the device.
Interference is possible in the vicinity of devices marked with the
following label.
18
BORCAD Medical a.s.
739 45 Fryčovice 673, tel.: +420 558 640 611, fax: +420 558 668 087 www.borcadmedical.com
Portable and mobile HF communication devices should not be used
closer than the recommended distance of d = 15 cm from any part of
the birthing bed, including the cables.
The birthing bed must be installed and put into operation in compliance with
information related to EMC provided in the accompanying documentation.
Portable and mobile HF communication devices may influence the electrical
medical device.
Manual and declaration of the manufacturer - electromagnetic radiation
The AVE birthing bed is intended for use in electromagnetic environment
specified below. The customer or user of the AVE birthing bed must ensure that
the bed is used in such environment.
High frequency radiation
CISPR 11
Group 1
The AVE birthing bed uses high
frequency energy only for its internal
functioning. Therefore, its high
frequency radiation is very low and is
not likely to cause any interference
with nearby electronic equipment.
High frequency radiation
CISPR 11
Class A
The AVE birthing bed is intended for
use in all facilities other than
households and buildings which are
directly connected to a public low-
voltage power supply network that
supplies buildings used for residential
purposes.
Harmonic
emissions
IEC 61000-3-2
Not
applicable
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
Not
applicable

19
BORCAD Medical a.s.
739 45 Fryčovice 673, tel.: +420 558 640 611, fax: +420 558 668 087 www.borcadmedical.com
Mechanical safety:
Before the mother in labor accesses the bed, the wheel brake must be
engaged!
The bed is not designed for mothers over 210 kg.
No objects that may collide with the bed may be located near it (furniture,
stands etc.)
Never enter the space under motor operated parts of bed (back, seat and
leg rests).
Before undertaking any resetting of the birthing bed check if no one is within
reach of the moving parts.
Before any resetting, check for kinked and/or tangled power cables
In case of failure of the control (permanent pressing of buttons or damage
to cable) the motor may be stopped by pressing the button for the opposite
direction of operation, and then it is necessary to unplug the power cable,
the batteries, and contact the service authority.
After setting the bed in place all brakes must be engaged so as to prevent
unwanted movement of the bed, e.g. during patient’s occupancy. When
setting the bed all four wheels have to be in contact with the floor and the
brakes engaged
Never step on chassis covers.
The birthing bed is not designed for permanent operation. - motors
can only be loaded up to the values defined on the label, i.e. 2 minutes
of operation followed by 18 minutes of stand by.

20
BORCAD Medical a.s.
739 45 Fryčovice 673, tel.: +420 558 640 611, fax: +420 558 668 087 www.borcadmedical.com
2.3 Setting the product
No objects (furniture, stands, etc.) may be placed near the bed that
could collide with it (minimum distance of 20 cm). Movable parts of the
chair must be treated with caution. Movement of these parts can cause
collision with other objects, which may lead to damage to parts of the
chair or injury of the patient, staff or any third person!
The power cable must not be connected through an extension cord and it
must not be connected to common multisocket inlets.
No input cable of this or other devices can lead over the surfaces of the bed
A and /or over the patient. When moving the bed avoid pinching/kinking of
any objects.
Pay attention to proper fixing of the approved accessories listed in the
manual
Installation is done by trained person; operating the birthing bed only
allowed by trained personnel for routine functions; operators must be trained
on setting sufficient height of the infusion stand.
Training will be performed by a representative of BORCAD Medical a.s.
(manufacturer) or its official dealer
Maximum weight of the patient 210 kg.
A battery is a component of every obstetric bed; it is fully charged and
flawless and allows for the operation of the bed for the time of approx. 3
minutes. The battery is recharged automatically from the control unit and
does not require any special maintenance. If the chair is not connected to
the network for more than one week, it is necessary to disconnect the
battery connector from the CB (Control box). With internal rechargeable
battery system for all functions of the bed.
This manual suits for next models
3
Table of contents
Other BORCAD Medical Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual