4 | ENDOFLOW® II –INSTRUCTION FOR USE
Contents
1. About this Instruction For Use.................................................................................................................................6
2. Description................................................................................................................................................................6
3. Symbols Used on Labeling........................................................................................................................................7
4. Indication for Use .....................................................................................................................................................9
5. Important Safety Information..................................................................................................................................9
6. Operating Instruction.............................................................................................................................................11
6.1. Device Description..........................................................................................................................................11
6.1.1. MEN01 & MEN01US ...............................................................................................................................11
6.1.2. MEN02P & MEN02PUS...........................................................................................................................11
6.2. Signification of Touch Screen Icons ...............................................................................................................12
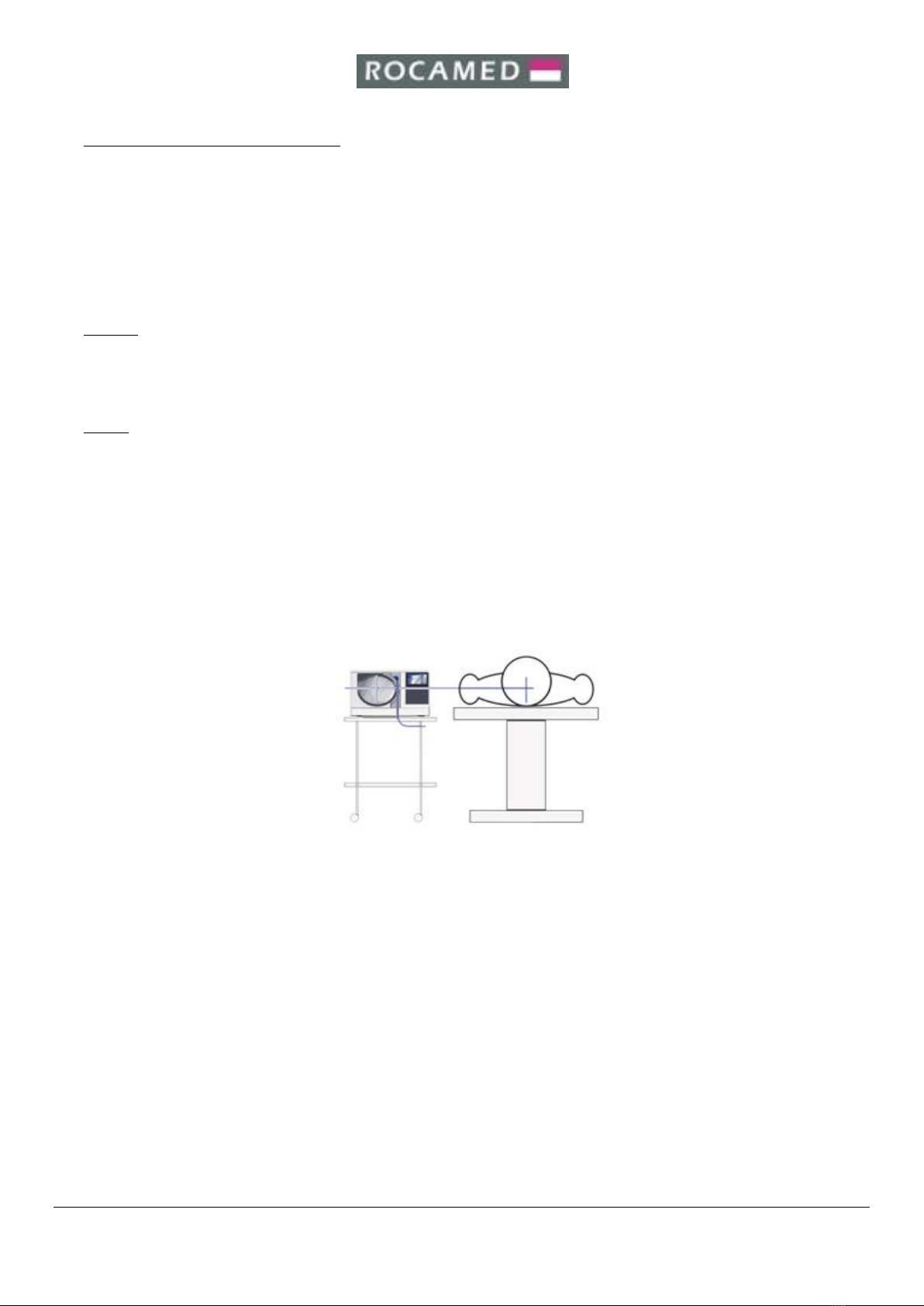
6.3. Set-Up for Use ................................................................................................................................................13
6.3.1. Electrical Connection..............................................................................................................................13
6.3.2. Pneumatic Connection ...........................................................................................................................13
6.3.3. Turn ON...................................................................................................................................................13
6.3.4. Warming .................................................................................................................................................13
6.4. Use the ENDFLOW® ........................................................................................................................................14
6.4.1. Irrigation .................................................................................................................................................14
6.4.2. Change the fluid Bag ..............................................................................................................................16
6.4.3. Suction ....................................................................................................................................................17
6.5. Peripherals......................................................................................................................................................18
6.6. After Use.........................................................................................................................................................18
6.7. Cleaning after Use ..........................................................................................................................................18
7. Troubleshooting .....................................................................................................................................................19
8. Limited Warranty ...................................................................................................................................................20
9. Service.....................................................................................................................................................................21
9.1. Warranty Service............................................................................................................................................21
9.2. Non-Warranty Work.......................................................................................................................................21
9.3. Expected Service Life......................................................................................................................................21
9.4. Destruction .....................................................................................................................................................21