Legende / Legend
SAP-Nr. / Plant PM code: 541512
Sprachvariante / Country code: 103
Version: 1
Datum / Date: 13.04.2018 PE
Abmessungen / Dimensions: 628 × 296 mm
Schriftgröße / Font size: 10 Pt
Zeilenabstand / Line spacing: 11 Pt
Seite / Page: 1/2
Druckbare Farben / Printing colours
Pantone Reex Blue C
Technische Information / Technical information
Kontur / Outline
Package leaflet: Information for the user
Lantus® SoloStar®
100 units/ml
solution for injection
in a pre-filled pen
insulin glargine
Talk to your doctor, pharmacist or nurse
before using Lantus.
Follow closely the instructions for posology,
monitoring (blood and urine tests), diet and
physical activity (physical work and exercise),
injection technique as discussed with your
doctor.
If your blood sugar is too low (hypoglycaemia),
follow the guidance for hypoglycaemia (see
box at the end of this leaflet).
Travel
Before travelling consult your doctor. You may
need to talk about
– the availability of your insulin in the
country you are visiting,
– supplies of insulin, needles etc.,
– correct storage of your insulin while
travelling,
– timing of meals and insulin administration
while travelling,
– the possible effects of changing to different
time zones,
– possible new health risks in the countries to
be visited,
– what you should do in emergency situations
when you feel unwell or become ill.
Illnesses and injuries
In the following situations, the management
of your diabetes may require a lot of care (for
example, adjustment to insulin dose, blood
and urine tests):
– If you are ill or have a major injury then
your blood sugar level may increase
(hyperglycaemia).
– If you are not eating enough your
blood sugar level may become too low
(hypoglycaemia).
In most cases you will need a doctor. Make
sure that you contact a doctor early.
If you have type 1 diabetes (insulin dependent
diabetes mellitus), do not stop your insulin
and continue to get enough carbohydrates.
Always tell people who are caring for you or
treating you that you require insulin.
Insulin treatment can cause the body to
produce antibodies to insulin (substances
that act against insulin). However, only very
rarely, this will require a change to your
insulin dose.
Some patients with long-standing type2
diabetes mellitus and heart disease or
previous stroke who were treated with
pioglitazone (oral anti-diabetic medicine used
to treat type2 diabetes mellitus) and insulin
experienced the development of heart failure.
Inform your doctor as soon as possible if
you experience signs of heart failure such as
unusual shortness of breath or rapid increase
in weight or localised swelling (oedema).
Children
There is no experience with the use of Lantus
in children below the age of 2years
Other medicines and Lantus
Some medicines cause changes in the blood
sugar level (decrease, increase or both
depending on the situation). In each case, it
may be necessary to adjust your insulin dose
to avoid blood sugar levels that are either too
low or too high. Be careful when you start or
stop taking another medicine.
Tell your doctor or pharmacist if you are
taking, have recently taken or might take any
other medicines. Before taking a medicine ask
your doctor if it can affect your blood sugar
level and what action, if any, you need to
take.
Medicines that may cause your blood sugar
level to fall (hypoglycaemia) include:
– all other medicines to treat diabetes,
– angiotensin converting enzyme (ACE)
inhibitors (used to treat certain heart
conditions or high blood pressure),
– disopyramide (used to treat certain heart
conditions),
– fluoxetine (used to treat depression),
– fibrates (used to lower high levels of blood
lipids),
– monoamine oxidase (MAO) inhibitors (used
to treat depression),
– pentoxifylline, propoxyphene, salicylates
(such as acetylsalicylic acid, used to relieve
pain and lower fever),
– sulfonamide antibiotics.
Medicines that may cause your blood sugar
level to rise (hyperglycaemia) include:
– corticosteroids (such as “cortisone” used to
treat inflammation),
– danazol (medicine acting on ovulation),
– diazoxide (used to treat high blood
pressure),
– diuretics (used to treat high blood pressure
or excessive fluid retention),
– glucagon (pancreas hormone used to treat
severe hypoglycaemia),
– isoniazid (used to treat tuberculosis),
– oestrogens and progestogens (such as in the
contraceptive pill used for birth control),
– phenothiazine derivatives (used to treat
psychiatric disorders),
– somatropin (growth hormone),
– sympathomimetic medicines (such as
epinephrine [adrenaline], salbutamol,
terbutaline used to treat asthma),
– thyroid hormones (used to treat thyroid
gland disorders),
– atypical antipsychotic medicines (such as
clozapine, olanzapine),
– protease inhibitors (used to treat HIV).
Your blood sugar level may either rise or
fall if you take:
– beta-blockers (used to treat high blood
pressure),
– clonidine (used to treat high blood
pressure),
– lithium salts (used to treat psychiatric
disorders).
Pentamidine (used to treat some infections
caused by parasites) may cause hypoglycaemia
which may sometimes be followed by
hyperglycaemia.
Beta-blockers like other sympatholytic
medicines (such as clonidine, guanethidine,
and reserpine) may weaken or suppress
entirely the first warning symptoms which
help you to recognise a hypoglycaemia.
If you are not sure whether you are taking
one of those medicines ask your doctor or
pharmacist.
Lantus with alcohol
Your blood sugar levels may either rise or fall
if you drink alcohol.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice
before taking any medicine.
Inform your doctor if you are planning to
become pregnant, or if you are already
pregnant. Your insulin dose may need to be
changed during pregnancy and after giving
birth. Particularly careful control of your
diabetes, and prevention of hypoglycaemia, is
important for the health of your baby.
If you are breast-feeding consult your doctor
as you may require adjustments in your
insulin doses and your diet.
Driving and using machines
Your ability to concentrate or react may be
reduced if:
– you have hypoglycaemia (low blood sugar
levels),
– you have hyperglycaemia (high blood sugar
levels),
– you have problems with your sight.
Keep this possible problem in mind in all
situations where you might put yourself and
others at risk (such as driving a car or using
machines). You should contact your doctor for
advice on driving if:
– you have frequent episodes of
hypoglycaemia,
– the first warning symptoms which help you
to recognise hypoglycaemia are reduced or
absent.
Important information about some of the
ingredients of Lantus
This medicine contains less than 1mmol
(23mg) sodium per dose, i.e. it is essentially
‘sodium-free’.
3. How to use Lantus
Always use this medicine exactly as your
doctor has told you. Check with your doctor or
pharmacist if you are not sure.
Although Lantus contains the same active
substance as Toujeo (insulin glargine
300units/ml), these medicines are not
interchangeable. The switch from one
insulin therapy to another requires medical
prescription, medical supervision and blood
glucose monitoring. Please, consult your
doctor for further information.
Dose
Based on your life-style and the results of your
blood sugar (glucose) tests and your previous
insulin usage, your doctor will
– determine how much Lantus per day you
will need and at what time.
– tell you when to check your blood sugar
level, and whether you need to carry out
urine tests,
– tell you when you may need to inject a
higher or lower dose of Lantus.
Lantus is a long-acting insulin. Your doctor
may tell you to use it in combination with a
short-acting insulin or with tablets used to
treat high blood sugar levels.
Many factors may influence your blood sugar
level. You should know these factors so that
you are able to react correctly to changes in
your blood sugar level and to prevent it from
becoming too high or too low. See the box at
the end of this leaflet for further information.
Use in children and adolescents
Lantus can be used in adolescents and
children aged 2 years and above. Use this
medicine exactly as your doctor has told you.
Frequency of administration
You need one injection of Lantus every day, at
the same time of the day.
Method of administration
Lantus is injected under the skin. Do NOT
inject Lantus in a vein, since this will change
its action and may cause hypoglycaemia.
Your doctor will show you in which area of
the skin you should inject Lantus. With each
injection, change the puncture site within the
particular area of skin that you are using.
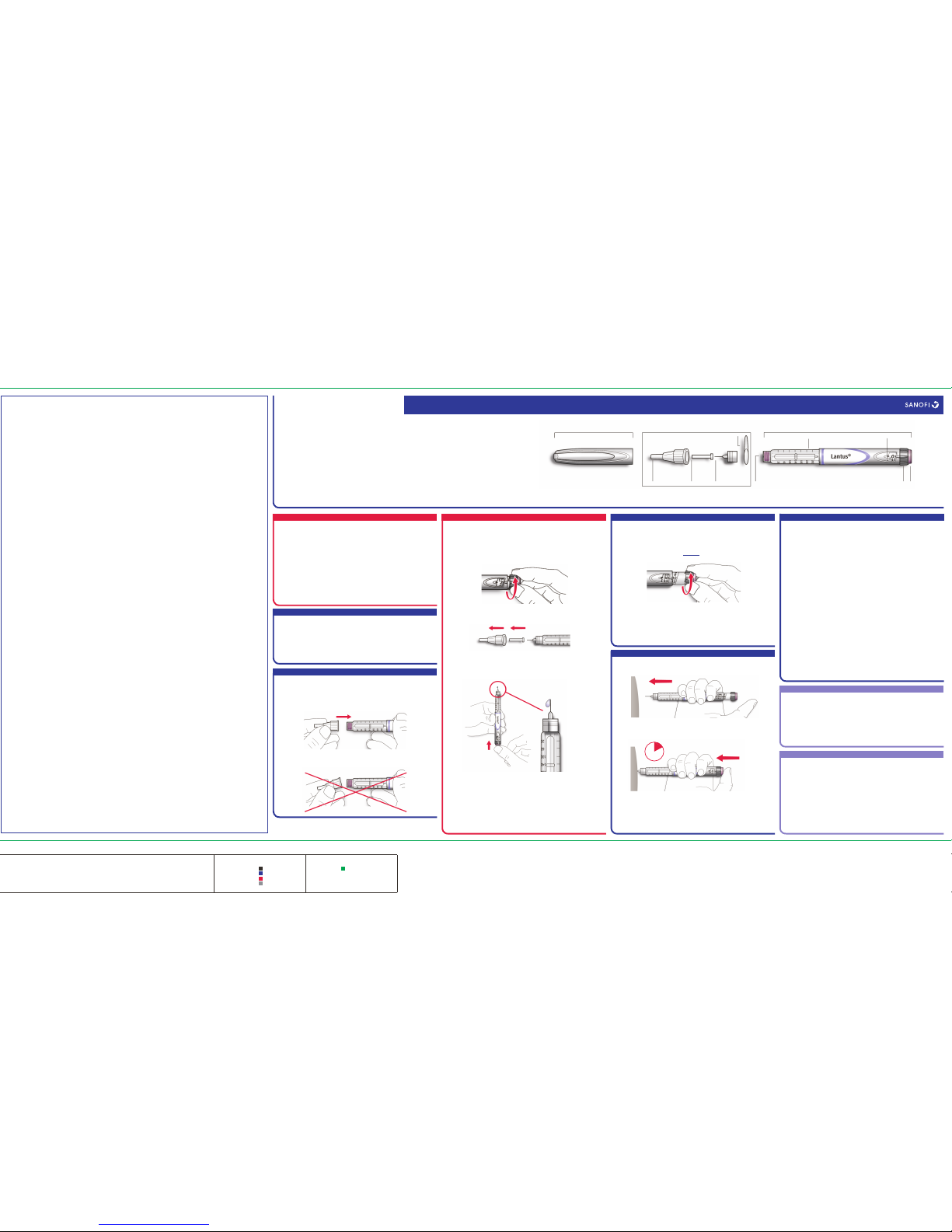
How to handle SoloStar
SoloStar is a pre-filled disposable pen
containing insulin glargine. Lantus in
pre-filled pen is only suitable for injecting
just under the skin. Speak to your doctor if
you need to inject your insulin by another
method.
Read carefully the “SoloStar Instructions
for Use” included in this package leaflet.
You must use the pen as described in these
Instructions for Use.
A new needle must be attached before each
use. Only use needles that are compatible for
use with SoloStar (see “SoloStar Instructions
for Use”).
A safety test must be performed before each
injection.
Look at the cartridge before you use the pen.
Do not use SoloStar if you notice particles in
it. Only use SoloStar if the solution is clear,
colourless and waterlike. Do not shake or mix
it before use.
To prevent the possible transmission of
disease, never share your pen with anyone
else. This pen is only for your use.
Make sure that neither alcohol nor other
disinfectants or other substances contaminate
the insulin.
Always use a new pen if you notice that your
blood sugar control is unexpectedly getting
worse. If you think you may have a problem
with SoloStar, consult your doctor, pharmacist
or nurse.
Empty pens must not be re-filled and must be
properly discarded.
Do not use SoloStar if it is damaged or not
working properly, it has to be discarded and a
new SoloStar has to be used.
Insulin Mix-ups
You must always check the insulin label
before each injection to avoid mix-ups
between Lantus and other insulins.
If you use more Lantus than you should
– If you have injected too much Lantus,
your blood sugar level may become too
low (hypoglycaemia). Check your blood
sugar frequently. In general, to prevent
hypoglycaemia you must eat more food and
monitor your blood sugar. For information
on the treatment of hypoglycaemia, see box
at the end of this leaflet.
If you forget to use Lantus
– If you have missed a dose of Lantus or if
you have not injected enough insulin,
your blood sugar level may become too
high (hyperglycaemia). Check your blood
sugar frequently. For information on the
treatment of hyperglycaemia, see box at the
end of this leaflet.
– Do not take a double dose to make up for a
forgotten dose.
If you stop using Lantus
This could lead to severe hyperglycaemia (very
high blood sugar) and ketoacidosis (build-up
of acid in the blood because the body is
breaking down fat instead of sugar). Do not
stop Lantus without speaking to a doctor, who
will tell you what needs to be done.
If you have any further questions on the use
of this medicine, ask your doctor, pharmacist
or nurse.
4. Possible side effects
Like all medicines, this medicine can cause
side effects, although not everybody gets them.
If you notice signs of your blood sugar
being to low (hypoglycaemia), take action
to increase your blood sugar level straight
away (see the box at the end of this leaflet).
Hypoglycaemia (low blood sugar) can be
very serious and is very common with
insulin treatment (may affect more than
1in 10people). Low blood sugar means that
there is not enough sugar in your blood.
If your blood sugar level falls too low, you
may pass out (become unconscious). Serious
hypoglycaemia may cause brain damage and
may be life-threatening. For more information,
see the box at the end of this leaflet.
Severe allergic reactions (rare, may affect up
to 1 in 1,000 people) - the signs may include
large-scale skin reactions (rash and itching
all over the body), severe swelling of skin or
mucous membranes (angiooedema),
shortness of breath, a fall in blood pressure
with rapid heart beat and sweating. Severe
allergic reactions to insulins may become
life-threatening. Tell a doctor straight away if
you notice signs of severe allergic reaction.
Common reported side effects (may affect
up to 1 in 10 people)
• Skin changes at the injection site
If you inject your insulin too often at the
same skin site, fatty tissue under the skin
at this site may either shrink (lipoatrophy,
Read all of this leaflet carefully including
the Instructions for Use of Lantus
SoloStar, pre- filled pen, before you start
using this medicine because it contains
important information for you.
– Keep this leaflet. You may need to read it
again.
– If you have any further questions, ask your
doctor, pharmacist or nurse.
– This medicine has been prescribed for you
only. Do not pass it on to others. It may
harm them, even if their signs of illness are
the same as yours.
– If you get any side effects, talk to your
doctor or pharmacist. This includes any
possible side effects not listed in this
leaflet. See section 4.
may affect up to 1 in 100 people) or thicken
(lipohypertrophy). The insulin may not work
very well. Change the injection site with each
injection to help prevent these skin changes.
• Skin and allergic reactions at the
injection site
(The signs may include reddening, unusually
intense pain when injecting, itching, hives,
swelling or inflammation). This can spread
around the injection site. Most minor
reactions to insulins usually disappear in a
few days to a few weeks.
Rare reported side effects (may affect up to
1 in 1,000 people)
• Eye reactions
A marked change (improvement or worsening)
in your blood sugar control can disturb your
vision temporarily. If you have proliferative
retinopathy (an eye disease related to
diabetes) severe hypoglycaemic attacks may
cause temporary loss of vision.
• General disorders
In rare cases, insulin treatment may also
cause temporary build-up of water in the
body, with swelling in the calves and ankles.
Very rare reported side-effects (may affect
up to 1 in 10,000 people)
In very rare cases, dysgeusia (taste disorders)
and myalgia (muscular pain) can occur.
Use in children and adolescents
In general, the side effects in children and
adolescents of 18 years of age or less are
similar to those seen in adults.
Complaints of injection site reactions
(injection site reaction, injection site pain) and
skin reactions (rash, urticaria) are reported
relatively more frequently in children and
adolescents of 18 years of age or less than in
adults.
There is no experience in children under
2years.
Reporting of side effects
If you get any side effects, talk to your doctor
or pharmacist. This includes any possible side
effects not listed in this leaflet.
United Kingdom
You can also report side effects directly via the
Yellow Card Scheme at:
www.mhra.gov.uk/yellowcard
or search for MHRA Yellow Card in the Google
Play or Apple App Store.
Ireland
You can also report side effects directly via
HPRA Pharmacovigilance, Earlsfort Terrace,
IRL - Dublin 2;
Tel: +353 1 6764971;
Fax: +353 1 6762517.
Website: www.hpra.ie;
Malta
You can also report side effects directly via
ADR Reporting
www.medicinesauthority.gov.mt/adrportal
By reporting side effects you can help provide
more information on the safety of this
medicine.
5. How to store Lantus
Keep this medicine out of the sight and reach
of children.
Do not use this medicine after the expiry date
which is stated on the carton and on the label
of the pen after “EXP”. The expiry date refers
to the last day of that month.
Not in-use pens
Store in a refrigerator (2°C-8°C). Do not freeze
or place next to the freezer compartment or a
freezer pack.
Keep the pre-filled pen in the outer carton in
order to protect from light.
In-use pens
Pre-filled pens in use or carried as a spare
may be stored for a maximum of 4 weeks
not above 30°C and away from direct heat or
direct light. The pen in use must not be stored
in the refrigerator. Do not use it after this time
period.
Do not throw away any medicines via
wastewater or household waste. Ask your
pharmacist how to throw away medicines
you no longer use. These measures will help
protect the environment.
6. Contents of the pack and other
information
What Lantus contains
– The active substance is insulin glargine.
Each ml of the solution contains 100 units
of insulin glargine (equivalent to 3.64 mg).
– The other ingredients of Lantus are: zinc
chloride, metacresol, glycerol, sodium
hydroxide (see section2 “Important
information about some of the ingredients
of Lantus”) and hydrochloric acid (for pH
adjustment) and water for injections.
What Lantus looks like and contents of the
pack
Lantus SoloStar 100 units/ml solution for
injection in a pre-filled pen, is a clear and
colourless solution.
Each pen contains 3 ml of solution for
injection (equivalent to 300 units). Packs sizes
of 1, 3, 4, 5, 6, 8, 9 and 10 pre-filled pens.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and
Manufacturer
Sanofi-Aventis Deutschland GmbH,
D-65926 Frankfurt am Main, Germany.
This leaflet was last revised in
April 2018
Other source of information
Detailed information on this medicine is
available on the European Medicines Agency
web site: http://www.ema.europa.eu/
For any information about this medicine,
please contact the local representative of the
Marketing Authorisation Holder.
Ireland
sanofi-aventis Ireland Ltd. T/A SANOFI
Tel: +353 (0) 1 403 56 00
Malta
Sanofi Malta Ltd., Tel: +356 21493022
United Kingdom
Sanofi, Tel: +44 (0) 845 372 7101
What is in this leaflet
1. What Lantus is and what it is used for
2. What you need to know before you use
Lantus
3. How to use Lantus
4. Possible side effects
5. How to store Lantus
6. Contents of the pack and other information
1. What Lantus is and what it is used
for
Lantus contains insulin glargine. This is a
modified insulin, very similar to human
insulin.
Lantus is used to treat diabetes mellitus in
adults, adolescents and children aged 2years
and above. Diabetes mellitus is a disease
where your body does not produce enough
insulin to control the level of blood sugar.
Insulin glargine has a long and steady
blood-sugar-lowering action.
2. What you need to know before you
use Lantus
Do not use Lantus
If you are allergic to insulin glargine or to
any of the other ingredients of this medicine
(listed in section6).
Warnings and precautions
Lantus in pre-filled pen is only suitable
for injecting just under the skin (see also
section3). Speak to your doctor if you need to
inject your insulin by another method.
<MAT>541512
<MAT>541512