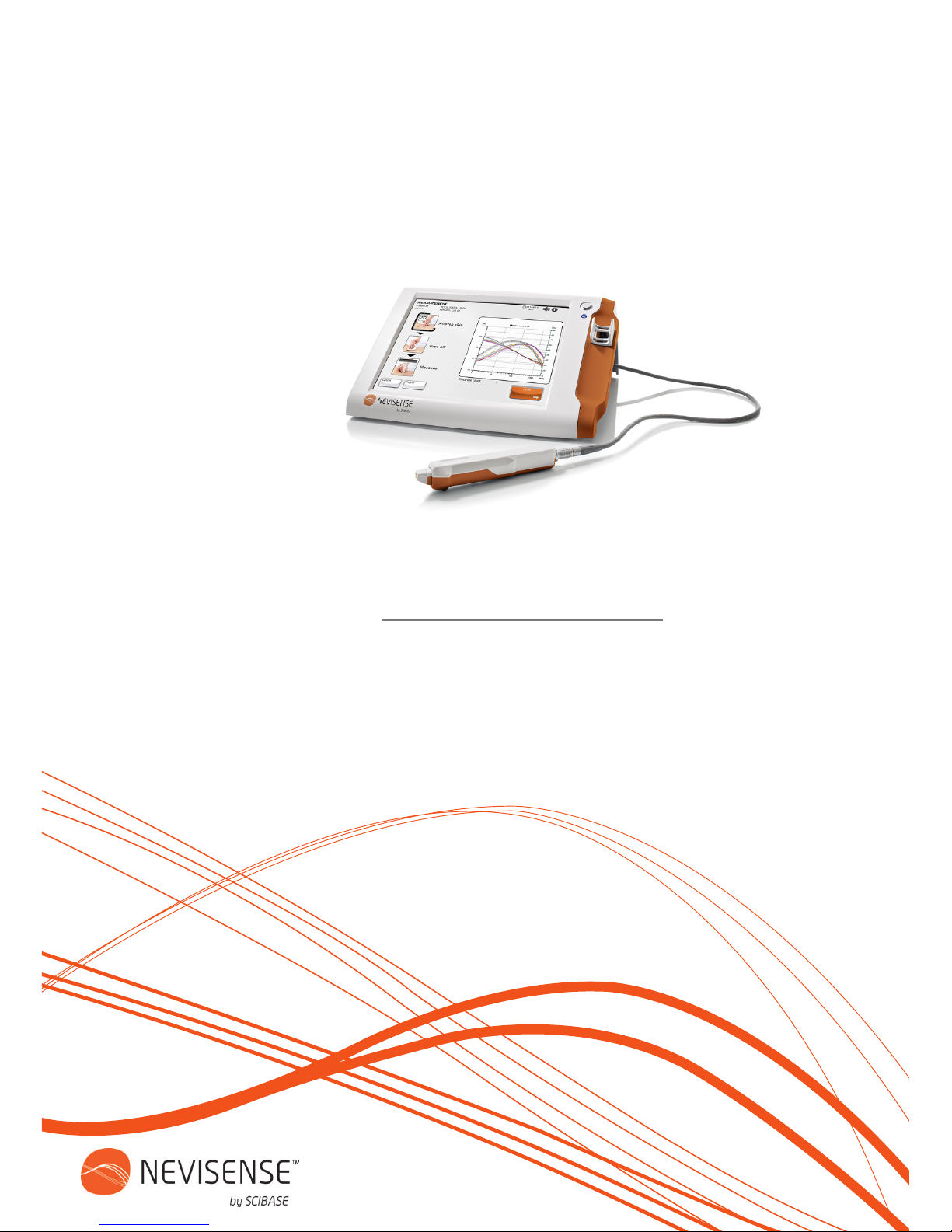
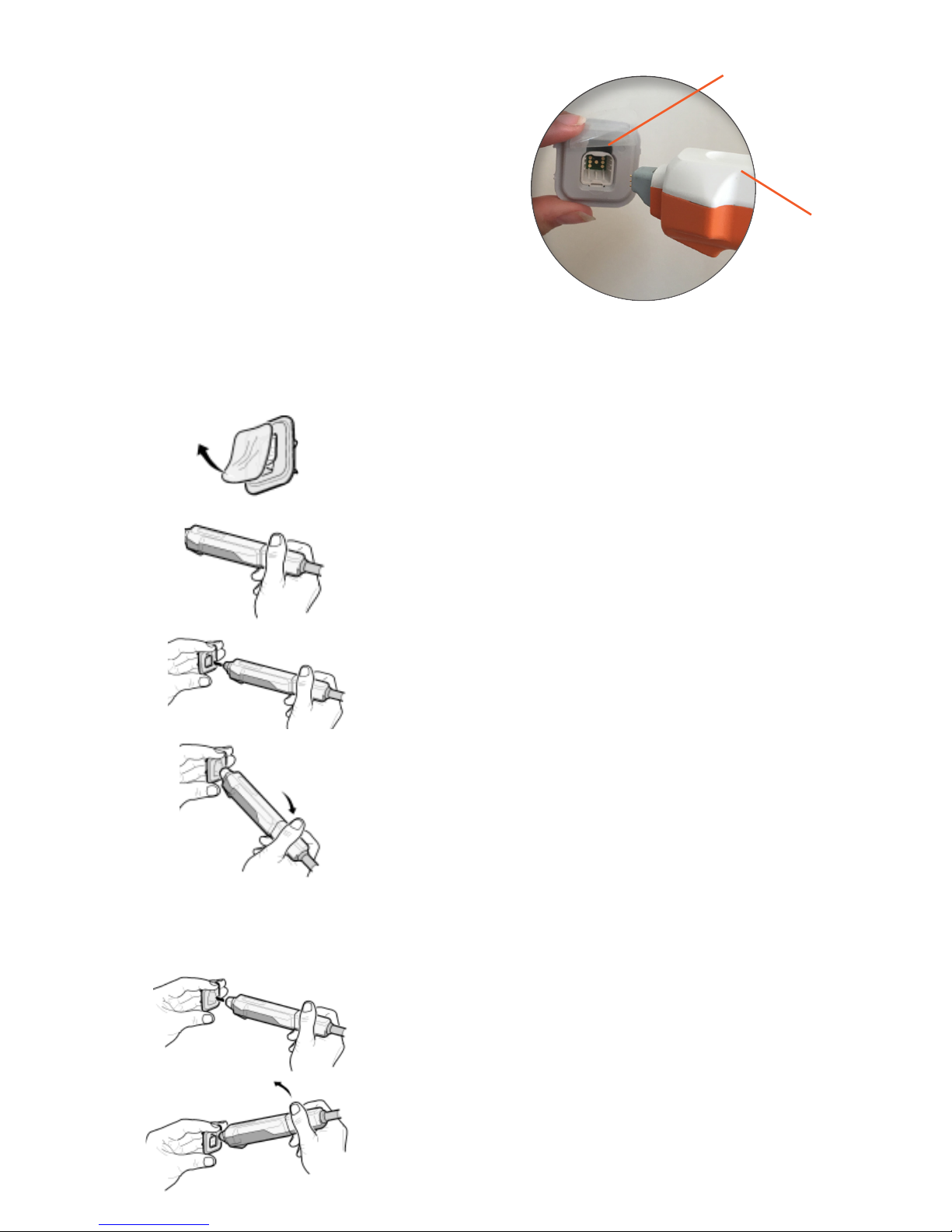
1. Remove the protective lm from the
electrode holder.
2. Hold the probe in the non-moveable part
close to the cable.
3. The probe should have the white side
upwards and the electrode holder the gap
upwards. Press the probe into the holder until
you hear a click. The click indicates that the
electrode is attached to the probe.
4. Angle the probe slightly downwards to
smothly release from the container.
REMOVE THE ELECTRODE
1. Press the electrode into an empty container
until you hear a click. The click indicates that
the electrode has snapped back into the
container.
2. To detach, angle the probe upwards before
pulling back.
Gap upwards
White side
upwards
ATTACH THE ELECTRODE
Warning! The disposable electrode
is for single person use. It has to be
replaced when an examination of a
patient is completed, and also
whenever Nevisense issues a
warning about a used electrode. This
is in order to minimize the risk of
spreading infections and to prevent the
electrode spikes from being worn out.
The electrode is approved for a maximum of 20 measurements.
3 (14)