Sedatelec Premio 30 laser duo User manual

HME-PREMIO30-ENG ENGLISH 1/28
User manual
Keep with your instrument
English

HME-PREMIO30-ENG ENGLISH 2/28
Thank you for choosing the Premio 30 laser duo!
Since it was formed, Sedatelec has based its work on
studies carried out in complementary medicine,
particularly those conducted by Dr P. Nogier, the father
of auriculotherapy and auriculomedecine, to offer
practitioners novel, highly reliable, high performance
instruments.
Nowadays we are developing and incorporating the values
of respecting patient, practitioner and the environment
into our products, consistent with the highest level
medical standards, so that you can practice comfortably
and effectively.
We are pleased to work with our excellent permanent
research partners and are also proud of our increasing
participation in sharing medical information between the
different people who work in the field. Please do not
hesitate to contact us for any further information by email
I hope you will be fully satisfied with your use of Premio
30 laser Duo. I am happy to hear from you and am open to
any ideas to advance your practice in this novel medical
approach.
Best wishes,
Thierry Garaboux, Chairman.

HME-PREMIO30-ENG ENGLISH 3/28
TABLE OF CONTENTS
SAFETY INSTRUCTIONS,RESIDUAL RISKS AND PRECAUTIONS FOR USE .......................... 4
INDICATIONS &CONTRAINDICATIONS ............................................................... 7
DESCRIPTION OF THE PREMIO 30 LASER DUO ...................................................... 9
HOW TO USE THE PREMIO 30 LASER DUO..........................................................11
TECHNICAL SPECIFICATIONS..........................................................................14
FREQUENCIES &PROGRAMMES.......................................................................15
MAINTENANCE ..........................................................................................15
TROUBLESHOOTING -RECYCLING ...................................................................16
APPENDIX 1: REGULATORY AND STANDARD RELATED INFORMATION...........................17
APPENDIX 2: PERIODICAL TECHNICAL SECURITY CHECKS ........................................19
APPENDIX 3: ELECTROMAGNETIC COMPATIBILITY.................................................20
APPENDIX 4: INSTRUMENT BOOKLET................................................................25

HME-PREMIO30-ENG ENGLISH 4/28
SAFETY INSTRUCTIONS,RESIDUAL RISKS AND PRECAUTIONS FOR USE
The Premio 30 laser duo is an infrared laser which emits a laser
beam at a rate of 905 nm.
The Maximum Permitted Emissions (MPE) are 0.269 J/m2for
the skin and 2.47*10-4 J/ m2for the cornea.
The "Laser danger" symbol, placed on the end of your device,
represents the "opening indicator plate" designating an
"opening for laser radiation"
Safety for the skin
The laser emission from the Premio 30 laser duo is under this
MPE and the beam can be directed onto the skin entirely safely
without causing harm.
Safety for the eyes
Important note: The instrument must never be used to treat
the globe of the eye.
The Nominal Ocular Danger Distance (NODD) is the distance
from which the light applied to the eye must be less than the
MPE for the cornea.
The NODD for the Premio 30 laser duo is 50 cm.
Whenever the laser is handled less than 50 cm from the eyes, it
is therefore recommended that anyone who could accidentally
look at the laser beam wears the protective glasses intended
for this purpose.

HME-PREMIO30-ENG ENGLISH 5/28
Precautions for use of the Premio 30 laser duo
You are recommended:
- Not charge it in stormy weather,
- Not to use it in an environment containing by
electromagnetic emissions outside of standard levels (eg:
close to a CT scanner or MRI),
- Not to use it if the casing is not intact,
- Not to use it in an explosive environment,
- Not to subject it to ionising irradiation (eg: X-rays),
- Not to store it at temperatures of under -20°C or over
50°C,
- Never to immerse it in a liquid.
The Premio 30 laser duo contains a Li-Ion technology battery
which must only be replaced by the staff instructed by
Sedatelec.
It is essential that only the charger designed for the
Premio 30 laser duo is used. Use of any other
charging system could damage the instrument
and may cause a high electrical discharge for the
user or a risk of explosion. The charger must remain accessible
to be disconnected from the power supply.

HME-PREMIO30-ENG ENGLISH 6/28
For safety reasons, the instrument is provided with a laser key,
which can restrict its use to authorised personnel.
The Premio 30 laser duo requires technical safety checks at
least every 24 months. These must be carried out by people
who have knowledge, equipment and experience to carry out
these checks (cf. appendix 2).
The Sedatelec technical department carries out all of the
appropriate technical tests on the Premio 30 laser duo.
Contact us…
Where applicable, Premio 30 laser duo should be installed and
used in accordance with the standard CAN/CSA-Z386-14: Safe
Use of Lasers in Health Care Facilities

HME-PREMIO30-ENG ENGLISH 7/28
INDICATIONS &CONTRAINDICATIONS
Indications
The Premio 30 laser duo is used for laser biostimulation
(coherent light) or locally on painful pathological areas of the
body.
The indications are those recognized for acupuncture, Ear
Acupuncture.
Its use requires skilled staff who have sufficient knowledge of
the indications, contraindications and medical risks of laser
biostimulation, in a medical situation.

HME-PREMIO30-ENG ENGLISH 8/28
Contraindications
- Direct contact with mucosal membranes or broken skin.
- Treatment of endocrine areas (particularly in children).
- Treatment of the globe of the eye.
- Treatment of abdomen in pregnant women.
- Treatment of the chest region in a patient with a cardiac
pacemaker.
- Exposure to sunlight or artificial UV light for 3 to 4 weeks
before treatment.
- Hypersensitivity to light at 905 nm.
-Previous use of anticoagulants and aspirin.
-Receipt of medicines when exposure to sunlight is
contraindicated.
-Receipt of oral isotretinoin medicines or anticoagulants.
-Past history of cheloid or hypertrophic scarring.
-Active infection/compromised immune system.
- Past history of herpes.
HME-PREMIO30-ENG ENGLISH 9/28
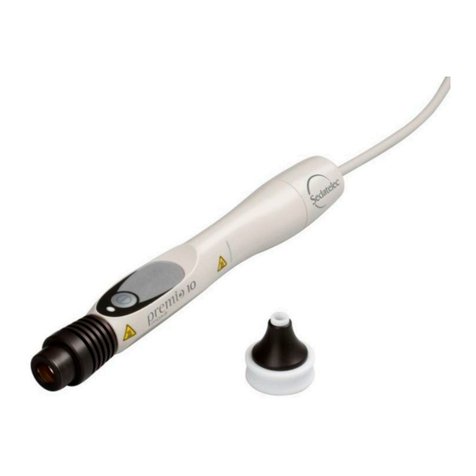
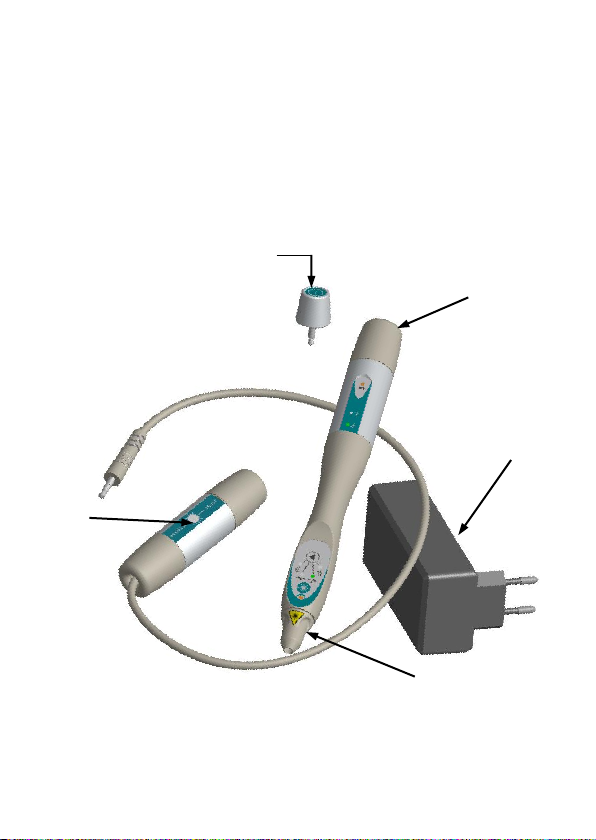
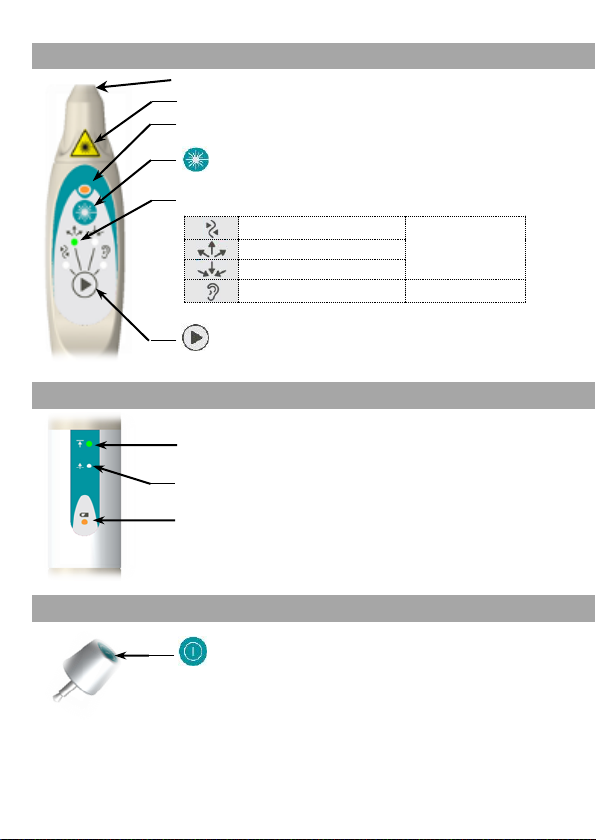
DESCRIPTION OF THE PREMIO 30 LASER DUO
The Premio 30 laser duo is supplied in a bag containing:
- A handpiece Premio 30 laser duo,
- A specific laser key,
- A test laser box,
- A battery charger,
- A pair of protective glasses with its manual,
- An instruction for use manual.
General description of the Premio 30 laser duo
Charger
Laser
Test
Laser key
Front
Rear
HME-PREMIO30-ENG ENGLISH 10/28
F
Fr
ro
on
nt
t
p
pa
an
ne
el
l
Laser beam output: invisible laser radiation
"Laser Danger" and "laser aperture" indicator
Laser emission indicator
Laser emission operating key
Laser stimulation programme indicator:
Harmonisation
Acupuncture
Dispersion
Tonification
Nogier frequencies scanning*
Ear Acupuncture
Type of laser stimulation selection key
R
Re
ea
ar
r
p
pa
an
ne
el
l
Superficial penetration indicator
Deep penetration indicator
Battery charge indicator
L
La
as
se
er
r
k
ke
ey
y
ON/OFF key and selection key for penetration
depth
*The application of Nogier frequencies with this device has not been authorised by
Health Canada for lack of sufficient clinical studies

HME-PREMIO30-ENG ENGLISH 11/28
HOW TO USE THE PREMIO 30 LASER DUO
Use of commands or settings or carrying out procedures
other than those described in this manual may result in
exposure to dangerous irradiation.
Before starting
a. To recharge the Premio 30 laser duo:
The Premio 30 laser duo cannot be used during charging.
•Remove the laser key,
•Insert the charger plug into the instrument,
•Connect the charger to the power supply.
Power indicator: (extract from the documentation *)
• Yellow: Charging (steady then flashing at the end of charging)
• Solid Green: Fully charged the battery.
• Flashing green: The PREMIO is not connected.
See charger manual * for:
• LED off or blinking red: Battery / charger fault
• Solid yellow + Flashing red: Temperature fault
*: See the attached manufacturer's manual or on
https://www.mascot.no/downloads/user-manuals/battery-chargers/ “chargers_li-
ion”)
In the event of a Battery fault: See chapter Troubleshooting.
b. To check correct operation of the Premio 30 laser duo:
-Remove the laser key,
-Introduce the test laser plug into the
instrument,
-Connect the laser key to the test laser,
-Press on the key.

HME-PREMIO30-ENG ENGLISH 12/28
-Press on the emission key => continuous
audible signal (if no signal, contact your
distributor or Sedatelec),
-Apply the laser beam contact with the test laser
unit.
If the audible signal remains unchanged, this means
that the energy emitted is not within the -20%/+20%
of nominal value band. You should then contact your distributor or
Sedatelec to have your instrument adjusted.
Note: After using, disconnect the Premio 30 laser duo test laser in
order that the battery does not discharge.
Starting and operating information
a. Insert the key into its socket on the back of the
instrument. Press on the ON/OFF key .
b. Checking the battery charge indicator:
- Slow flashing: recharge recommended
- Fast flashing: the instrument may stop at any
time; the battery is in urgent need of
recharging.
Important comment: when switched on, the Premio 30 laser duo
returns to the configuration for the stimulation mode in last use, in
superficial penetration.
HME-PREMIO30-ENG ENGLISH 13/28
c. Select the stimulation mode: press on the key to select
the desired programme: harmonisation, dispersion,
tonification or Nogier frequency* scanning.
d. In Acupuncture positions, to select the treatment depth
press on the key to alternatively select laser penetration
depth for superficial or deep points.
Note: In the Ear Acupuncture position, depth is selected automatically.
e. Press on the key:the light indicator and a continuous
audible signal inform the user that the laser is being
emitted. After 30 seconds, a beep is emitted and the laser
emission is automatically stopped.
f. To stop the beam being emitted during the cycle of 30
seconds, press on the key again.
Stopping the instrument
a. Long press on the key.
Note: The instrument also stops automatically after 2 minutes without
use.
b. Remove the key.
*The application of Nogier frequencies with this device has not been authorised by
Health Canada for lack of sufficient clinical studies

HME-PREMIO30-ENG ENGLISH 14/28
TECHNICAL SPECIFICATIONS
Manufacturer Sedatelec
Name of the device Premio 30 laser duo
Type Infrared laser
Emission features
Type of laser diode InGaAs/GaAs
Wavelength 905 nm
Crest power 16.1 / 43.7 W (typical, not tested)
Energy per pulse 1.125 / 3.6 µJ
Energy per 30 sec 15 / 50 mJ
Emission frequency 100 –10000 Hz
73 –4672 Hz
Beam divergence 9° x 25°
Electrical supply
Internal battery Li-Ion 3.6V 850 mAh
Li-Ion charger 90-264V ~/ 47-63Hz
Mechanics
Handpiece 213mm x 25mm
Weight of handpiece 111g
Operating conditions
Temperature between 10°C and 35°C
Relative humidity < 70 %
Atmospheric pressure between 70 kPa and 106 kPa
Storage and transport
Temperature between -20° and 50°C
Relative humidity < 90 %
Atmospheric pressure between 70 kPa and 106 kPa
Eye protection
Required optical density >=2
Energy/pulse lighting 8.2*10-3 J/ m2to 10cm
MANUFACTURED IN FRANCE

HME-PREMIO30-ENG ENGLISH 15/28
FREQUENCIES &PROGRAMMES
Harmonization
Acupuncture
Dispersion
Tonification
Nogier frequencies scanning*
from A to G (73 to 4672 Hz)
Auricular
acupuncture
Form of stimulation :
Harmonization
Dispersion
Tonification
100Hz à 10kHz
et 10kHz à 100Hz
10kHz à 100Hz
100 à 10kHz
MAINTENANCE
We recommend routine disinfection of parts which may
come into contact with patients.
Your Premio 30 laser duo does not require specific
maintenance and can be cleaned with a cloth soaked in
soapy water, 70°C alcohol or with a cold disinfectant.
Note: The instrument is not watertight and must not be
sprayed or immersed in a fluid.
When not used for long periods of time, it is recommended
that the instrument is stored with the battery charged and

HME-PREMIO30-ENG ENGLISH 16/28
that at least 1 charge/discharge cycle is carried out every 6
months.
The laser protective glasses can be cleaned with a soft
cloth soaked in soapy water.
If the lenses are perforated, scratched, damaged or change
in colour, or if the frame is damaged, the protective glasses
must no longer be used and must be replaced.
You must not attempt to change or repair the
parts inside the instrument or its accessories.
Any such attempt will invalidate the guarantee and may cause
serious risks to yourself, those around you and your patients.
TROUBLESHOOTING -RECYCLING
If the instrument does not function correctly, return it
completely in its original bag to your retailer or to Sedatelec
with your comments.
When disposing of your Premio 30 laser duo, note
that it contains electronic components and you
should observe the current instructions for your
country.

HME-PREMIO30-ENG ENGLISH 17/28
APPENDIX 1: REGULATORY AND STANDARD RELATED INFORMATION
Classification:
The Premio 30 laser duo is a class IIa medical device according
to directives 93 /42 EEC and 2007/47/EC.
Applicable standards:
- NF EN ISO 14971 :2013
- EN 60601-1: 2012
- IEC 60601-1-2 :2014
- IEC 60601-2-22 : 2012
- IEC 60825-1 :2014
- NF EN1041 :2008.
- ISO 15223-1 :2017
S
SY
YM
MB
BO
OL
LS
S
U
US
SE
ED
D
Manufacturer
"Refer to the manual / instruction booklet"
Dispose with electrical waste (directive DEEE)
EC Mark ensuring compliance with directives
93/42/EEC and 2007/47/EC awarded by TÜV SÜD
Product Service. The notified body, registration
number 0123.
Maximum and minimum limits of :
Atmospheric pressure
Hygrometry
Temperature

HME-PREMIO30-ENG ENGLISH 18/28
LABELS USED
Positioning: on the device : :
Serial number of the device
Battery: Li-ion 3.6V 0.85Ah
Charger: DC 4.2V 0.6A
Positioning:: On food
Product designation
QR code + digits: UDI identifier *
: Device reference
: Name and address of
manufacturer
: Date of manufacture
Positioning: On the box
:UDI identifier *
:Medical Device
Positioning: Towards the laser output of
the device
Laser danger (+ indication by the
tip of the warning "laser opening")
Positioning: In this manual
Warning: "laser opening"
Laser radiation is emitted by the
diode located at the end of the tip
Positionnement : in the case
Warning: "invisible laser radiation.
Dangerous beam exposure. Class 3B
laser device according to IEC 60825-
1.
Positioning: On the "laser test" box
: batch number of the "laser
test"
* UDI: Unique identifier of the product

HME-PREMIO30-ENG ENGLISH 19/28
APPENDIX 2: PERIODICAL TECHNICAL SECURITY CHECKS
Reminder: This laser device must have technical safety checks
every 24 months.
Visual examination:
Of the overall device,
Of the labelling: identification and warning labels,
commands and control markings, current method
of use etc.
Operating control depending on method of use:
Starting,
Setting commands and indicators,
Confirming the frequencies emitted,
Checking timer.
Checking emitted energy:
Check crest power emitted by each pulse
Technical safety check:
Laser Test check
Examination of the laser safety key
We would thoroughly recommend that you perform these
checks.
Sedatelec is at your service to do these!

HME-PREMIO30-ENG ENGLISH 20/28
APPENDIX 3: ELECTROMAGNETIC COMPATIBILITY
Appropriate electromagnetic environment.
The Premio 32D laser is suitable for use in all facilities including
domestic and those directly connected to a low voltage public
electrical supply network and domestic building supplies.
The Premio 32D laser uses RF energy only for its internal
operation. As a result its RF emissions are therefore very low
and are not liable to cause interference with a neighbouring
electrical instrument.
WARNING: Avoid using this instrument next to other machines
as it may cause incorrect operation. If use is required, this and
other instrument should be monitored to confirm their correct
operation.
Table of contents
Other Sedatelec Medical Equipment manuals
Popular Medical Equipment manuals by other brands

HEBU medical
HEBU medical ECO II Series Operating and service manual
Drive DeVilbiss Healthcare
Drive DeVilbiss Healthcare Sidhil CHE03 Instructions for use
Vortice
Vortice CA WE Instruction booklet

Chattanooga Group
Chattanooga Group INTELECT LEGEND Series user manual

Physio Control
Physio Control LIFEPAK 15 Service Process Document

Flaem
Flaem Rhino Clear User instruction manual