Shenzhen Hexin Zondan Medical Equipment ZD120E Series Operator's manual
ZD120D/ ZD120E User Instruction Manual
Fourth Edition: October 2021
Version: V1.4
I
Product Information
Product model: ZD120E Series
Product name: Patient Monitor
Manufacturer
Shenzhen Hexin ZONDAN Medical Equipment Co., Ltd.
Registered/Production address:
Floor 14, Block D, Dianlian Technology Building, the Crossing between South Circle
Road and South Fuli Road ,Guangming District,518106 Shenzhen PEOPLE'S
REPUBLIC OF CHINA
Tel: +86 755 26865970 Fax: +86 755 26860497
Edition
Fourth Edition: October 2021
Version: V1.4
MEDHealth supplies (Pty) Ltd
All Rights Reserved.
Distributor
MEDHealth Supplies (Pty) Ltd
Corner of Barbara and North Reef Road
Elandsfontein, Henville, 1429, Germiston, Johannesburg, Gauteng, South Africa
Reg No.: 2021/385832/07 Telephone: +27 10 013 3010
E-mail: admin@medhealthsup.com
Regulatory and Safety Specifications
Standard
The product is made under the ISO13485 quality system certified by TUV PS. The
product has passed the CE certification.
Declaration
The ZD120E Patient Monitor is a Class IIb device and complies with the requirements of
the Council Directive 93/42/EEC concerning medical devices and carries CE-marking
accordingly.
Authorized EU Representative
Shanghai International Holding Corp.GmbH (Europe)
Eiffestrasse 80, 20537 Hamburg, Germany
Tel: 0049-40-2513175 Fax: 0049-40-255726
ZD120E User Instruction Manual
Fourth Edition: October 2021
Version: V1.4
II
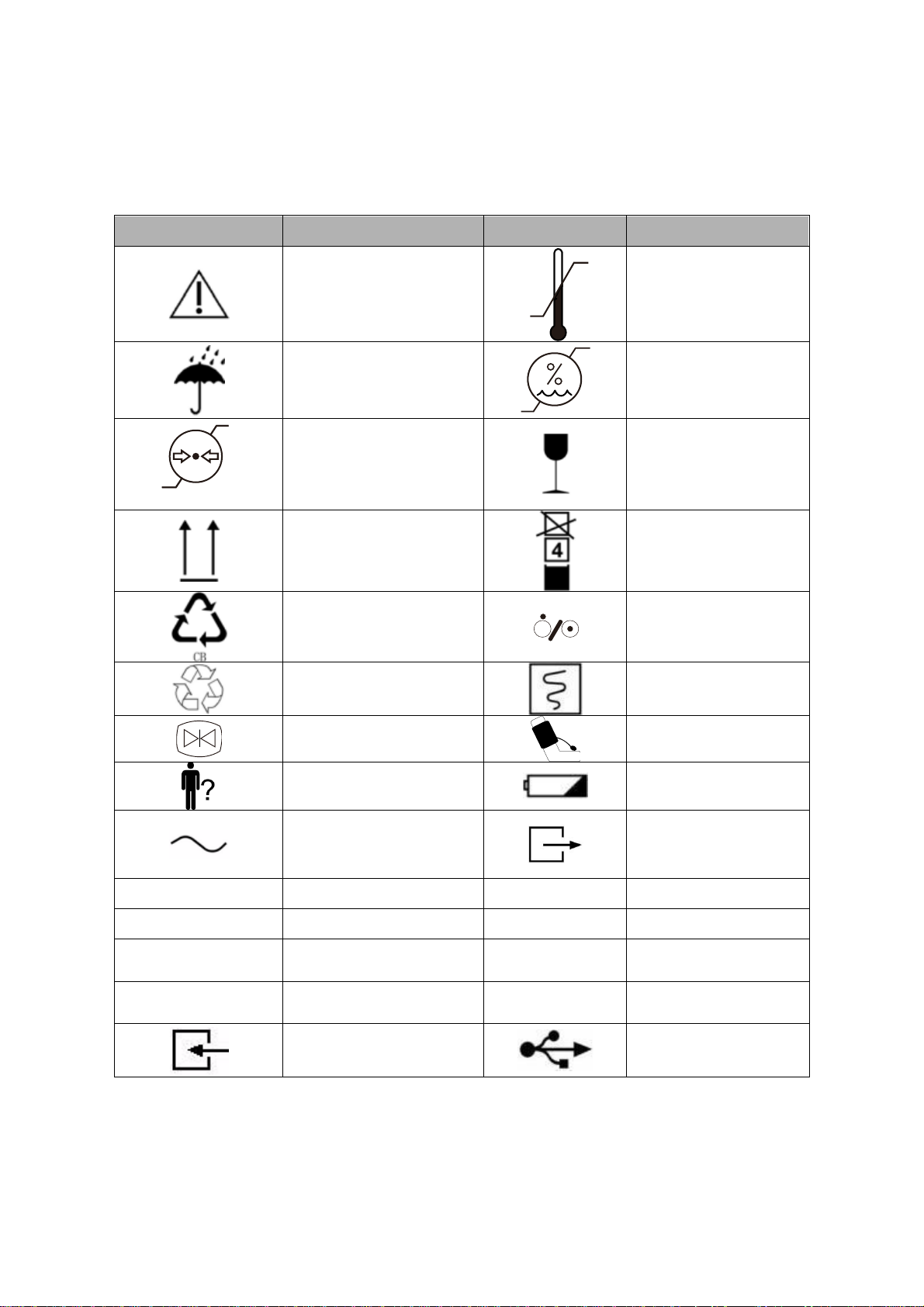
Explanation of Symbols
The following symbols appear on the monitor and packaging.
Table 1 Monitor and Packaging Symbols
Symbol
Description
Symbol
Description
Caution, consult
accompanying
documents
-2 0℃
5 5℃
Temperature limitations
Keep dry
10%
93%
Humidity limitations
22kPa
107.4kPa
Atmospheric pressure
limitations
Fragile, handle with care
Keep upright
Maximum stacking
Recovery
Power On
key
Recyclable
Record key
Waveform Freeze key
NIBP key
Patient Key
Charging LED
AC Power LED
Gas Output
CO2
CO2connector
ECG/RESP
ECG connector
NIBP
NIBP cuff hose connector
SpO2
SpO2probe connector
TEMP1
TEMP1 connector
IBP1
IBP1 transducer
cable connector
TEMP2
TEMP2 connector
IBP2
IBP2 transducer
cable connector
Gas input
USB port

ZD120D/ ZD120E User Instruction Manual
Fourth Edition: October 2021
Version: V1.4
III
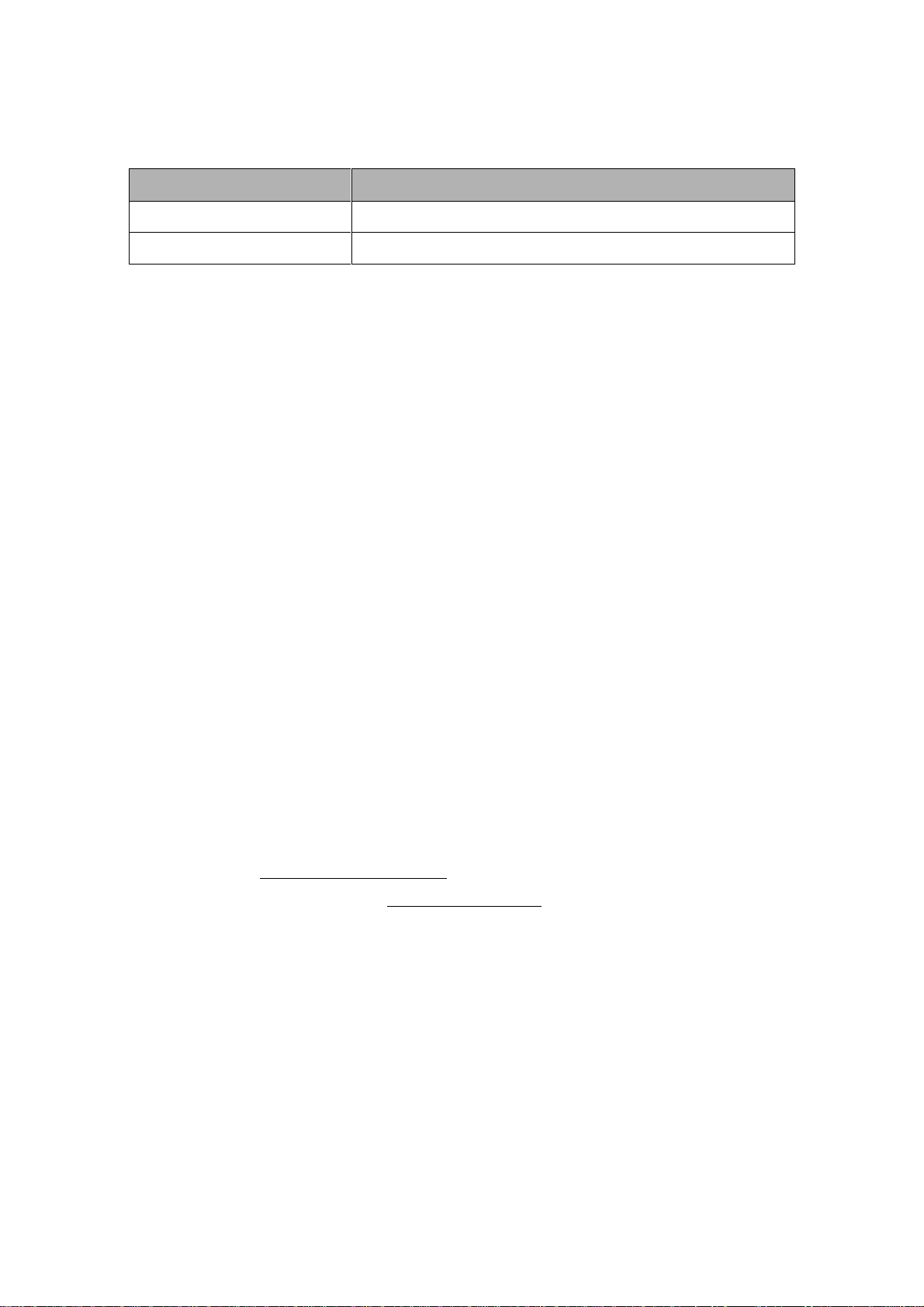
Table 1 Monitor and Packaging Symbols (continue)
Symbol
Description
Symbol
Description
BF applied part: including F
applied part (float/insulation)
BF applied part:
including F applied part
(float/insulation) and
defibrillation-proof
function.
CF applied part: including F
applied part (float/insulation)
and defibrillation-proof
function.
Fuse
Ethernet port
Protective
grounding
Video output
Dangerous Voltage
Equipotential grounding
Manufacturer address
Date of manufacture
Configuration number
Serial Number
EU representatives
Batch code
Consult Instructions for
Use
Catalogue number
Compliance to WEEE
standard
Protective grade
Safety Standards
The following table describes the safety standards of the ZD120E Patient Monitor.
Table 2 Safety Standards
Parameter
Specification
Protection class
Class I, anti-shock, externally and internally powered
equipment, per IEC 60601-1
Degree of protection
Type CF and BF defibrillator-proof: per IEC 60601-1
Degree of noxious-liquid proof as IPX1
Anti-shock degree as combination of BF and CF applied part
According to the degree of safety of application in the presence of
a flammable anesthetic mixture with air or with oxygen or nitrous
oxide, the equipment is not suitable for use in the presence of a
flammable anesthetic mixture with air or with oxygen or nitrous
oxide
ZD120E User Instruction Manual
Fourth Edition: October 2021
Version: V1.4
IV
Table 2 Safety Standards (continue)
Parameter
Specification
Sterilization and disinfection
As recommended by manufacturer
Mode of operation
Continuous
Product Support and Warranty Information
The manufacturer warranties the ZD120E Patient Monitor for 12 months. Keep the
packing case for transport, storage, or maintenance.
The manufacturer is responsible for the safety, reliability, and performance of the monitor
when the:
•Product is assembled, upgraded, altered, or maintained by authorized service
representatives.
•Location where the product is placed is that of a typical hospital environment.
•Product is used according to this guide.
The manufacturer is not responsible for damage to the monitor when the:
•Damage is caused by:
–Improper operation.
–Improper connection of the monitor to other devices.
–Accidental impact.
–Water/liquid damage.
•Monitor is altered without written authorization from MEDHealth Supplies.
•Serial number of the monitor is removed or becomes illegible.
After-Sales Service
The South African call center can be reached during the following time: Monday–
Friday (except public holidays)
1. Contact your local MEDHealth representative.
2. For further support, contact MEDHealth’ Customer Service Department.
Customer Service Department of MEDHealth Supplies (Pty) Ltd, Corner of Barbara and
North Reef Road, Elandsfontein, Henville, 1429, Germiston, Johannesburg, Gauteng,
South Africa
E-mail: technical@medhealthsup.com
Portal for technical support: www.medhealthsup.com
Tel: +27 10 013 3010
Before calling for technical support, note the following information:
•Model and serial number of the monitor
•Monitor problem
The international call center can be reached during the following time: Monday–
Friday (except Chinese statutory holidays)
BJT 08:30–12:00, 13:30–18:00 (GMT+8)
Tel: +86 755 26865970/8037
Fax: +86 755 26860497

ZD120D/ ZD120E User Instruction Manual
Fourth Edition: October 2021
Version: V1.4
V
E-mail: service1@zondan.com
Service and support are available in Chinese and English only. Before calling for service,
note the following information:
•Model and serial number of the monitor
•Monitor problem
Safety Conventions
The manual uses the following conventions for Notes, Cautions, and Warnings.
Note —A Note calls attention to an important point in the text.
Caution A Caution calls attention to a condition or possible situation that could damage or
destroy the product or the user’s work.
Warning A Warning calls attention to a condition or possible situation that could cause injury
to the user and/or patient.
Safety Requirements
Note —The safety indications in this chapter apply to general monitor use. Safety
indications in other chapters apply to specific monitor measurements.
Follow the instructions in this user manual when using the monitor. However,
conventional medical practices always supersede this document. Significance of safety
requirements set forth here in this manual is not in order of reading sequence.
Warning The monitor is not for home use.
The monitor is only for use on one patient at a time. The monitor is not for
diagnostic or therapeutic use.
The monitor is not an apnea monitor. The respiration measurement does not
recognize obstructive and mixed apneas —it only indicates when a user-defined
time has elapsed since the last detected breath.
Anyone who connects to additional equipment to the signal input port or signal
output port configures a medical system and is therefore responsible to ensure that
the system complies with the requirements of standard IEC 60601-1. Never
permanently install equipment connected to the signal input or output ports. If in
doubt, contact MEDHealth Supplies.
To avoid mixture, clear all historical data of the last patient before monitoring a
new patient. Ensure that the patient monitor is in good working condition and is
placed in proper position before clinical use.
Never rely exclusively on the alarm system for patient monitoring. You must
periodically check that monitor alarms are working properly. The most reliable
method of patient monitoring combines close, personal surveillance along with the
correct operation of the monitor.
For pacemaker patients, rate meters may continue to count the pacemaker rate
during occurrences of cardiac arrest or come arrhythmia. Do not rely entirely upon
Table of contents
Other Shenzhen Hexin Zondan Medical Equipment Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual












