Siem Nova 6110-A3 SIEM Operating instructions

USER and MAINTENANCE MANUAL 6110 A3 and 6110 A4
Manufacturer: Siem Nova S.r.l. Legal and operational offices 200 7 ROZZANO (Milano) MADE IN ITALY
Product: SURGICAL ASPIRATOR MOD: 6110-A3 SIEM and 6110-A4 JUNIOR
Updated: 2018-03-19 reproduction forbidden Pag: 1 di 10
ASSISTANCE
Read the instruction manual before using the device.
This manual must be kept with the device for later reference.
SIGNS AND SYMBOLS
This symbol indicates the
compliance with the
essential requirements of
the Directive 93/42/ EEC
of 14 June 1993
concerning medical
devices. Reference IMQ
Spa - Milan
This symbol
indicates an
insulation class I
device.
This symbol indicates
to follow instructions
for use.
This symbol indicates the
date of production (four
digits for the year and two
digits for the month).
This Symbol
indicates a type B
applied part.
This symbol indicates
to consult instructions
for use.
This symbol indicates the
manufacturer.
This symbol
indicates alternate
current
This symbol indicates
the foot switch.
This symbol indicates that
interferences may occur in
the vicinity of equipment
marked with this symbol
This symbol
indicates a single
use device. Do not
reuse the device.
This symbol indicates a
CAUTION or WARNING
associated with the
device.
This symbol indicates the
humidity limitation for
operation, transport and
storage
This symbol
indicates the
atmospheric
pressure limitation
for operation,
transport and
storage.
This symbol indicates
the temperature
limitation for
operation, transport
and storage.
This symbol indicates to
keep the device away from
sunlight
This symbol
indicates to keep the
device dry.
IP33
This symbol indicates
the protection against
harmful effects due to
ingress of solid foreign
objects and against
harmful effects due to
the ingress of water.
This symbol indicates do
not dispose the device
together with unsorted
municipal waste (for EU
only).
This symbol indicates
do not immerse in
water or other liquid
PRESSURE MEASURE UNIT CONVERSION TABLE
Legenda: bar, KPa, cm Hg, cm H O are all measure unit of pressure (vacuum)
bar KPa cm Hg cm H2O
1 bar 1 100 75.006 1019.72
1 KPa 0.01 1 0.75006 10.1972
1 cm Hg 0.133 1.333 1 13.595
1 cm H2O 0.09 0.0009 0.07355 1
1APPLICATION
The suction equipment 6110-A3 SIEM and 6110-A4 JUNIOR are approved exclusively for the use as described in this user and
maintenance manual. Siem Nova can only warranty the safe functioning of the equipment when the 6110-A3 SIEM and 6110-A4
JUNIOR are used with the Siem Nova accessories.
Please read and observe these warning and safety instructions before use the device.
Please note that these instructions for use are a general guide for the use of the product.
Medical matters must be addressed by a physician.

USER and MAINTENANCE MANUAL 6110 A3 and 6110 A4
Manufacturer: Siem Nova S.r.l. Legal and operational offices 200 7 ROZZANO (Milano) MADE IN ITALY
Product: SURGICAL ASPIRATOR MOD: 6110-A3 SIEM and 6110-A4 JUNIOR
Updated: 2018-03-19 reproduction forbidden Pag: 2 di 10
WARNINGS: The products are designed for the safety care of patient and operator but some warnings must be
followed.
REMARKS: The users must be able to read, understand and follow directions provided in this manual and provided by
the physician. If you are dependent on the device for airway suctioning and a breakdown can lead to a critical
situation, you must have another suitable device available.
1. Only use the 6110-A3 SIEM and 6110-A4 JUNIOR suction pump on the person for whom they were ordered and only for
theirs intended use.
2. The equipments must be used by instructed and qualified operators.
3. If you note changing in performances of the equipments, please contact immediately authorized service center.
4. Do not perform the therapy without your physician supervision.
5. The connecting tubing delivered with the device must always be connected to a sterile catheter or suitable accessories as
prescribed by your physician.
6. Do not modify the equipments without manufacturer authorization.
7. The operators must avoid the contact with blood or body liquids.
. If the filter or the over flow valve does not operate and the liquids go inside the equipments remove immediately the power
supply connection.
9. Do not use the equipments in presence of flammable anesthetic mixture with air, oxygen or nitrogen protoxid.
10. The use of mobile telephones, cordless telephones and other communication equipments can affect the DOMI suction
pump. A safety distance of min. 1 m to the 6110-A3 SIEM and 6110-A4 JUNIOR equipment is recommended.
11. Keep the power supply cord away from hot surfaces.
12. Keep the power cords away from moisture.
13. Never remove the mains plug out of the fixed mains socket by pulling on the power supply cord!
14. Never leave the devices unattended when they are operating.
15. The devices must stand upright during use.
16. Never use the devices while bathing or showering
17. Do not use extension cords with 6110-A3 SIEM and 6110-A4 JUNIOR equipment.
1 . Keep the power supply cord where you will not fall or trip over it.
19. The power cords or the tubes (for their length) could be strangle hazardous.
20. Never place the power supply cord or tubing around your neck.
21. Some equipment components for their small size could be ingested.
22. Keep the pump clean and dry.
23. Never place the pump in water or liquids.
24. Never touch with wet hands electrical parts.
25. If the pumps get wet, rub with dry towel. Do not dry in a microwave.
26. Do not touch the suction pumps when they have fallen into water. Unplug device immediately from main power supply
27. Keep the equipments away from children and pets.
2 . Keep the equipments protected from direct sunlight.
29. Keep the equipments away from heat source
30. Prevent the suction pumps from falling to the floor.
31. Don’t forget to take a spare canister / tubing and filters with you.
32. Check the general conditions of delivery package of the 6110-A3 SIEM and 6110-A4 JUNIOR for completeness and the
presence of all accessories supplied.
33. In the case of allergic reaction due to contact with the materials of this device, contact your doctor.
DESCRIPTIONS OF THE DEVICE
.1 Introduction
These suction pumps 6110-A3 SIEM and 6110-A4 JUNIOR from Siem Nova are quality suction pumps that combine easy
handling and cleaning with safety features to ensure optimal operation.
. Intended use
The 6110-A3 SIEM and 6110-A4 JUNIOR suction pumps are indicated for aspiration and removal of secretions, body
fluids and foreign objects especially from a patient's air way. It could be a respiratory support system in the nasal,
pharyngeal and tracheal areas of pediatric and adult patients.
The electrical surgical aspirators described can be used with following purpose:
•Mod. 6110 A3 SIEM: electrical surgical aspiration and removal of patient's air way secretions
•Mod. 6110 A4 JUNIOR: electrical surgical aspiration and removal of patient's air way secretions.
•Mod. 6110 A4 CURET JUNIOR: suitable for use in gynaecological operating theatre for endo-uterine aspiration as hystero-
suction device.
•Mod. 6110 A4 LIPO JUNIOR: electrical surgical aspiration suitable in cosmetic surgery and liposuction.
.3 Contraindications
The devices are not suitable for use for prolonged suctioning (thoracic or gastro-intestinal etc).
.4 Intended user
The 6110-A3 SIEM and 6110-A4 JUNIOR should only be operated by properly instructed users.
.5 Service life
The service life of the devices is five years. (except accessories)
.6 Package contents
Please ask your supplier in case of missing parts or for additional accessories
(*) The disposable cannulas are not included and should be purchased separately. The Cannulas must be in
compliance with medical device directive EEC 93/42
USER and MAINTENANCE MANUAL 6110 A3 and 6110 A4
Manufacturer: Siem Nova S.r.l. Legal and operational offices 200 7 ROZZANO (Milano) MADE IN ITALY
Product: SURGICAL ASPIRATOR MOD: 6110-A3 SIEM and 6110-A4 JUNIOR
Updated: 2018-03-19 reproduction forbidden Pag: 3 di 10
The package contains:
6110-A3 SIEM
INCLUDED ACCESSORIES SET
60750.1
Socket, cable and connector
n. 1
60972
Liner jar 2000 ml
n. 1
60 15
Polycarbonate lid with overflow valve
n. 1
90020
Clamp holder for jar
n. 1
90312
Clamp bracket for jar
n. 1
66974
Antibacterial/viral filter 11x11 mm
n. 1
66609
Hydrophobic “LIQUID STOP” Filter
n. 1
66233
Silicon tube 7x13mm
2 m
OPTIONAL
1016
Pneumatic footswitch
60973
Polycarbonate jar 3000 ml
90060
Polycarbonate jar 5000 ml with cap and
overflow valve (Graduated 4000 ml)
20221
Over flow jars with floating over flow valve
Disposable bags and jars
6110-A4 JUNIOR
INCLUDED ACCESSORIES SET
60750.1
Socket, cable and connector
n. 1
60972
Liner jar 2000 ml
n. 1
60 15
Polycarbonate lid with overflow valve
n. 1
90020
Clamp holder for jar
n. 1
90312
Clamp bracket for jar
n. 1
66974
Antibacterial/viral filter 11x11 mm
n. 1
66609
Hydrophobic “LIQUID STOP” Filter
n. 1
66233
Silicon tube 7x13mm
2 m
OPTIONAL
1016
Pneumatic footswitch
661 0
4 arms aluminum trolley
60973
Polycarbonate jar 3000 ml
90060
Polycarbonate jar 5000 ml with cap and
overflow valve (Graduated 4000 ml)
20221
Over flow jars with floating over flow valve
60777
Changeover suction switch
Disposable bags and jars
6110-A4 LIPO JUNIOR
INCLUDED ACCESSORIES SET
60750.1
Socket, cable and connector
n. 1
60972
Liner jar 2000 ml
n. 1
60 15
Polycarbonate lid with overflow valve
n. 1
90020
Clamp holder for jar
n. 1
90312
Clamp bracket for jar
n. 1
66974
Antibacterial/viral filter 11x11 mm
n. 1
66609
Hydrophobic “LIQUID STOP” Filter
n. 1
66233
Silicon tube 7x13mm
2 m
OPTIONAL
1016
Pneumatic footswitch
661 0
4 arms aluminum trolley
60973
Polycarbonate jar 3000 ml
90060
Polycarbonate jar 5000 ml with cap and
overflow valve (Graduated 4000 ml)
20221
Over flow jars with floating over flow valve
60777
Changeover suction switch
Disposable bags and jars
Reusable liposuction cannulas and handle
6110-A4 CURET JUNIOR
INCLUDED ACCESSORIES SET
60750.1
Socket, cable and connector
n. 1
60972
Liner jar 2000 ml
n. 1
60 15
Polycarbonate lid with overflow valve
n. 1
90020
Clamp holder for jar
n. 1
90312
Clamp bracket for jar
n. 1
66974
Antibacterial/viral filter 11x11 mm
n. 1
66609
Hydrophobic “LIQUID STOP” Filter
n. 1
66233
Silicon tube 7x13mm
2 m
OPTIONAL
1016
Pneumatic footswitch
661 0
4 arms aluminum trolley
60973
Polycarbonate jar 3000 ml
90060
Polycarbonate jar 5000 ml with cap and
overflow valve (Graduated 4000 ml)
20221
Over flow jars with floating over flow valve
92000
Set of 7 inox intrauterine aspiration curettes
(Ø 5, 6, 7, , 9, 10, 11 mm) and 1 handle
60777
Changeover suction switch
Disposable bags and jars
NOTE: These accessories sets are our standard accessories but the configuration can be different on
customer request
CAUTIONS: The 6110-A3 SIEM and 6110-A4 JUNIOR device are to be set up in such a way, that a separation
from the mains supply can be easy.
WARNING: These equipments must be used only by medically trained persons who have been adequately
trained in suction procedures and in the use of aspirators. Wear gloves for all operations.

USER and MAINTENANCE MANUAL 6110 A3 and 6110 A4
Manufacturer: Siem Nova S.r.l. Legal and operational offices 200 7 ROZZANO (Milano) MADE IN ITALY
Product: SURGICAL ASPIRATOR MOD: 6110-A3 SIEM and 6110-A4 JUNIOR
Updated: 2018-03-19 reproduction forbidden Pag: 4 di 10
VACUUM GAU
G
E
VACUUM
REGULATOR
NAMEPLATE
FOOTSWITCH
SOCKET
POWER
CORD
EQUIPOTENTIAL
SOCKET
SOCKET WITH
FUSES
PNEUMATIC
FOOTSWITCH
MAIN SWITCH
ANTIVIRAL
ANTIBACTERIAL
FILTER
ANTIBACTERIAL
VIRAL FILTER
11
x
11mm
SILICONE TUBE
WITH YELLOW PIPE
C
ONNECTIONS
PNEUMATIC
FOOTSWITCH
4 arms aluminum trolley
4 wheels with brake diam
80 mm
Hydrophobic “LIQUID
STOP
”
Filter
.7 Description
3. ELECTRICAL DIAGRAM AND COMPONENTS DESCRIPTION
6110 A3 SIEM – 6110 A4 JUNIOR with footswitch
REFERENCE DESCRIPTION
I 101 Three-pin switch
H1… Connectors
M Motor-driven compressor
P IPF Socket with fuses door
IPP MIC1 Pneumatic footswitch
I RF Footswitch relay
SPG Main light
SPF Functioning light
VENT Impeller
6110 A3 SIEM - 6110 A4 JUNIOR
REFERENCE DESCRIPTION
I 01 Bipolar switch with light
H1… Connectors
M Motor-driven compressor
P IPF Socket with fuses door
VENT Impeller
USER and MAINTENANCE MANUAL 6110 A3 and 6110 A4
Manufacturer: Siem Nova S.r.l. Legal and operational offices 200 7 ROZZANO (Milano) MADE IN ITALY
Product: SURGICAL ASPIRATOR MOD: 6110-A3 SIEM and 6110-A4 JUNIOR
Updated: 2018-03-19 reproduction forbidden Pag: 5 di 10
4 PREPARATION FOR USE
4.1 CHECKS BEFORE USE
before use always check:
a. damage of the power cord or main socket,
b. obvious equipments damage safety defects
c. proper functioning of the device.
d. Liquid presence in the filter or in the VACUUM tube
e. Liquid stop filter not occluded.
Check all accessories before use:
a. Canister and lid for cracks. Replace if necessary.
b. Tubing for cracks, brittle areas and that connectors and caps are firmly attached. Replace if necessary
c. Sterile accessories must be checked on the integrity of the packaging before use. Replace if necessary.
d. Not sterile and reusable accessories must be cleaned and disinfected before use
CAUTION: Do not use tubing or other sterile accessories if the sterile packaging is damaged. Do not reuse
disposable use or sterile accessories.
4. Assembly of the canister
a. check if the overflow valve is fixed on the lid of the canister and if the float (with sealing) is inside the cage.
b. Mount the lid on the canister. Check the “V” seal. Place connectors, vacuum tube, hydrophobic filter and liquid stop filter
(if present).
OVERFLOW SAFETY
It is placed under the Lid of jars and in each overflow jars. It is made by a float and a support cage.
Its function is to stop the aspiration when reached the maximum liquids level.
4.3 Positioning the canister on the 6110-A3 SIEM and 6110-A4 JUNIOR suction pump
a. Put the canister on the equipment on the suitable rail or hose.
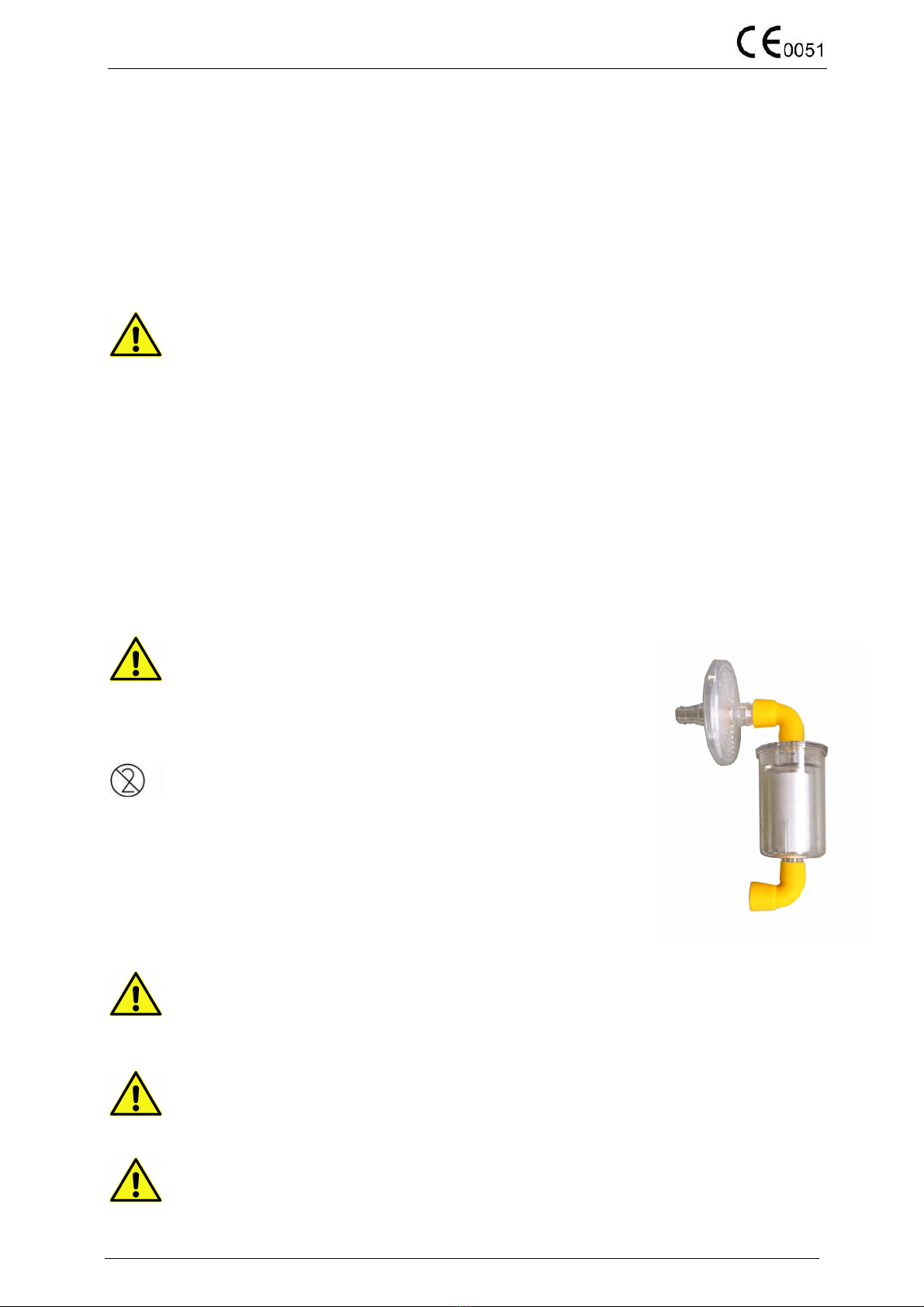
4.4 Positioning the Hydrophobic and antibacterial filter
1. Insert the Hydrophobic filter on the 6110-A3 SIEM and 6110-A4 JUNIOR rubber adapter, connect the vacuum tube on
the filter and on the lid (vacuum hose).
2. Connect the patient tube to the lid and to the available accessories (catheters or cannulas or other following your
physician indications) for better fixing you could use the yellow hose adapter available on lid.
3. Close with the yellow rubber caps the not used patient connections.
WARNING: Always check the presence of liquids or other materials in the
hydrophobic filter and/ or in the vacuum tube, if liquids or other visible contamination
are present, the FILTER must be replaced immediately due to the risk of pressure
build-up and possible filter membrane leakage (at vacuum level of -0.69 bar the
membrane breaks after 10 minutes). The filter membrane leakage allows the liquid to
go inside the pump and to damage it.
CAUTION: These filters have been designed, tested and manufactured
exclusively for disposable use and for a period of use not exceeding 4 hours.
4.5 Positioning the Hydrophobic filter LIQUID STOP
1. Insert the liquid stop filter by the hose on the lid (please check the right position of the vacuum
SIDE) then connect by the tube to the antibacterial filter.
Combined with the antibacterial filter the Hydrophobic filter "Liquid stop" effectively protect the
suction pump not only by the body liquid over flow but also by the small drops in the air (aerosol)
due to the suction operation; this small drops cannot be stopped by the over flow floating valve
on the lid and usually are stopped by the antibacterial filter but often the operator do not replace
this filter as indicated and sometimes this means to break the own filter and to contaminate and damage the pump. The LIQUID
stop filter must be replaced when dirty and/or occluded (by the small liquid drops)
WARNING: Always check the presence of liquids or other materials in the liquid stop filer filter, if liquids or
other visible contamination are present, the FILTER must be replaced immediately
5 OPERATING INSTRUCTIONS
CAUTIONS: The 6110-A3 SIEM and 6110-A4 JUNIOR device are to be set up in such a way, that a separation
from the mains supply can be easy.
WARNING: These equipments must be used only by medically trained persons who have been adequately
trained in suction procedures and in the use of aspirators. Wear gloves for all operations.

USER and MAINTENANCE MANUAL 6110 A3 and 6110 A4
Manufacturer: Siem Nova S.r.l. Legal and operational offices 200 7 ROZZANO (Milano) MADE IN ITALY
Product: SURGICAL ASPIRATOR MOD: 6110-A3 SIEM and 6110-A4 JUNIOR
Updated: 2018-03-19 reproduction forbidden Pag: 6 di 10
5.1 Connect 6110-A3 SIEM and 6110-A4 JUNIOR to main power
1. Check the equipment before use following the instruction in chapter 4 "Preparation for use".
2. insert the power cord plug in the 6110-A3 SIEM and 6110-A4 JUNIOR socket and then to the mains socket available.
to activate the equipment with the foot switch (if present or requested):
1. insert the foot switch tube on the foot switch socket
2. set the ON/OFF/ON switch to the symbol indicated, then press the pneumatic pad with the foot;
3. Pressing a second time will turn off the machine.
5. Functional check
1. Press the ON/OFF switch to switch on the equipment (the light on control panel will turn ON).
2. (If present) to activate the foot switch push the on/off switch on proper position (the light on control panel will turn ON).
3. (If present) test the jar switch by turning it left or right.
4. Set vacuum regulator to position «max.».
5. Close the patient tube with a finger and check if there is suitable suction. If there is suction, proceed with following step, if
not, see chapter “Troubleshooting".
6. Use accessories and vacuum setting as prescribed by a physician.
5.3 Changing vacuum level
Set vacuum regulator to the necessary position keeping close the patient tube and by turning the knob of the vacuum regulator
clockwise to arise the vacuum level and counterclockwise to reduce the vacuum. Read on vacuum gauge the set value.
5.4 JAR SELECTION (if present)
The operators can collect liquids aspirated into one or into the other jar simply by turning the switch for the selection.
5.5 Placing out of operation after use
1. Press the ON/OFF switch to switch off the pump
2. Remove the power cord from the main socket and then from the equipment AC socket
3. Remove canister from the equipment pulling the external yellow rubber stripes
4. For transportation of used canister always seal the lid with the caps.
5. Hang a clean canister (if required)
6. Clean and disinfect the equipment as described in chapter 7 "Cleaning guidelines”
7. For disposable material follow local after-use guidelines
WARNING: On a daily basis or latest when the fluid level reaches 70% of maximum canister capacity or the
over flow valve stops suction into the canister the canister should be emptied, cleaned and sterilized. Always check
the presence of liquids inside the filter and the vacuum tube. Empty canister and dispose of wasted material in
accordance with local guidelines
EN
6 TROUBLESHOOTING
6.1 If the equipment is not running
Check if:
1. the equipment is switched on.
2. the main voltage is correct
3. the plugs of the power cord are both correctly inserted in the mains socket and in the equipment.
6. the equipment does not suction strongly enough
Check if:
1. the vacuum regulator is set to the correct and prescribed suction level.
2. if the jar switch is present check if you are using the right jar.
3. the tubing is not defective and/or broken and/or obstructed and/or bended. If necessary, replace.
4. all tubing connections are tight. If necessary, fix them stronger.
5. the canister and/or the lid have no cracks, brittle areas, discolouration. If necessary, replace.
6. there is no liquids or other material inside the VACUUM tube or filters and the filters aren’t occluded.
WARNING: IF LIQUIDS REACH THE FILTERS IT WILL STOP THE AIR FLOW
CAUTIONS: If the fault cannot be rectified, please contact assistance.
7 CLEANING AND STERILIZATION GUIDELINES
7.1 General notes
1. Follow the cleaning instructions given by your local regulations.
2. Wear protective gloves for cleaning / disinfection.
3. Dispose fluids such as blood and secretions and the parts contaminated with them according to local guidelines.
7. Washing water
Use only the purest quality of water for cleaning. Water hardness is a serious consideration since deposits left on medical products
may not be properly removed. Use demineralized water in order to reduce this problem.
7.3 Disposable products
These are single use products not intended to be reused. Reuse could cause loss of mechanical, chemical and / or biological
characteristics. Reuse could cause cross contamination.

USER and MAINTENANCE MANUAL 6110 A3 and 6110 A4
Manufacturer: Siem Nova S.r.l. Legal and operational offices 200 7 ROZZANO (Milano) MADE IN ITALY
Product: SURGICAL ASPIRATOR MOD: 6110-A3 SIEM and 6110-A4 JUNIOR
Updated: 2018-03-19 reproduction forbidden Pag: 7 di 10
7.4 Disassembly
Separate all individual parts before cleaning and disinfecting.
7.5 Cleaning the suction unit, power cord and stripes
WARNINGS: Before cleaning the device, pull the mains plug out of the wall socket. Do not immerse the
equipments in water.
1. remove the power cord,
2. remove the canister
3. use a soft sponge or tissue with cold disinfectant solution (i.e. solution containing up 2% sodium hypochlorite) avoiding liquid
penetration inside the unit and power cord
4. avoid any operation causing liquid penetration inside the equipment or inside the power cord plugs
5. follow the cold disinfectant solution manufacturer instruction and check the compatibility between the solution and the case
material of unit (ABS), use a soft clean cloth to dry
EN
7.6 Cleaning canister, lid and tubing (for reusable materials only)
1. Remove the lid of reusable canister.
2. disassembly the cage of the overflow valve and the overflow valve
3. Clean components in hot water (60–70 °C) containing a detergent with a pH range between 6.0 and .0 only, in order to
avoid damage.
4. Soak all parts thoroughly with warm, soapy water (60–70 °C) or in enzymatic detergent for 1–5 minutes.
5. Remove visible dirt with a cleaning tool (i.e. general-purpose cleaning brush, such as pipe cleaners or non-abrasive lint
clothes).
6. Rinse thoroughly in clear water.
7. Dry.
. Check the parts for visible dirt and repeat these steps if necessary.
7.7 Sterilization jar, lid and tubing
WARNING: The procedure must be done by the qualified personnel after every use: personnel should have
individual protections such as coats, masks, gloves, screens, glasses, peaks, anti-sprinklings, etc....: the procedure is
intended to reduce the microbial load, provided for protecting the operator from HIV contamination, to limit the risk
of infection. This procedure requires that all reusable material came into contact with potentially infectious
materials, shall, after the use, be immediately immersed in a solution of Phenols for 30 minutes (see handbook
disinfectants).
All the material reusable after the decontamination and before the sterilization process must be thoroughly washed
in all its parts (see point 7.6)
1. sterilize the items into an autoclave (cycle 15'-121° C) or into an autoclave with ethylene oxide, cycle at 37°C (after ethylene
oxide sterilization material has to be degassed for 4 hours),
2. in alternative to point 1) cool sterilizing or dipping could be made. Follow instructions of the suitable chemical product
manufacturer for using it.
3. After 30 sterilizing cycles it is recommended to check the wholeness of the container, lid, tube and vacuum connectors
8 PREVENTIVE MAINTEINANCE
The 6110-A3 SIEM and 6110-A4 JUNIOR suction pump should not require preventive maintenance if used following the indication
of this manual.
If a 6110-A3 SIEM and 6110-A4 JUNIOR pump fails within the warranty period due to a manufacturing defect, it will be replaced.
REMARK: The defective equipment will need to be returned to the supplier.
8.1 Daily checkup (by user)
This check could quickly verify if the equipment is suitable for the use and it takes few minutes.
1. Connect the 6110-A3 SIEM and 6110-A4 JUNIOR to a main power supply.
2. Switch on the 6110-A3 SIEM and 6110-A4 JUNIOR, pushing the on/off switch (if the foot switch is present push the switch on
position l), the equipment must work smoothly without changing of motor turns, not normal noises or vibrations
3. Close the VACUUM tube, turn in clock wise the Vacuum regulator and check that the VACUUM level reaches at least the value
indicated on following tables (less 5% max).
4. With the VACUUM tube open, turn in clock wise the Vacuum regulator and check that the VACUMM level will decrease towards
0 (zero) (due to load loss could be accepted a residual vacuum level of 40-50 mbar).
5. Switch off the equipment.
6. Check if the filter is clean and no liquid or other contamination inside. If the color of the filter is not white it must be replaced,
a dirty filter means not good performances of the 6110-A3 SIEM and 6110-A4 JUNIOR (see the chapter 4.4).
7. If necessary, replace filters and disposable bag (if used).
8. 6/1 MONTHS CHECK UP (after warranty period)
This check verifies if the equipment is in compliance with the original productions features and so suitable to be used. This check
should be done by specialized operators or authorized service companies, following the functional test these operators or
companies should do a safety electrical test in compliance with IEC 60601-1 and issue a final report.
TEST LIST TO BE DONE ON THE EQUIPMENTS:
1. Check the pump: the Maximum Vacuum should be not less than the value indicated on following tables (less 5% max), and
there are not noises or vibrations.
2. Check the vacuum regulator rotating it in both the senses and verifying smooth working operations.
3. Check the mechanical integrity of the aluminium case, the penetration of external material could damage the equipment or
create danger to the operators.
4. Check if the labels are still present and readable.
5. Never open the device, for this technical operation please contact authorized service center only

USER and MAINTENANCE MANUAL 6110 A3 and 6110 A4
Manufacturer: Siem Nova S.r.l. Legal and operational offices 200 7 ROZZANO (Milano) MADE IN ITALY
Product: SURGICAL ASPIRATOR MOD: 6110-A3 SIEM and 6110-A4 JUNIOR
Updated: 2018-03-19 reproduction forbidden Pag: 8 di 10
6. Check the Vacuum gauge. With the equipment off the hand must indicate 0 mbar (zero)
7. Check the integrity of the canisters, connections and tubing.
. If necessary, replace filters and disposable bag (if used)
9. Before declaring the equipment in compliance with the manufacturer features make a safety electrical test (IEC 60601-1). For
making this test ask to the authorized service center and/or to the manufacturer.
Use disposable or spare parts supplied from the manufacturer only, the compliance of disposable or spare
parts could be confirmed by the manufacturer only.
Issue and properly archive a suitable report for the check done.
The operator must consider that the use of the 6110-A3 SIEM and 6110-A4 JUNIOR in high quote, in this
condition the value of vacuum could reduced, do not use the 6110-A3 SIEM and 6110-A4 JUNIOR over 3000 mt from
the sea level (984 ft).
9 TECHNICAL SPECIFICATIONS
9.1 Transport/Storage conditions
The 6110-A3 SIEM and 6110-A4 JUNIOR pump and accessories must remain in the packaging for storage and stored at
a temperature range from –25 °C to +70 °C (–13 °F to +15 °F).
9. Operating temperature
The 6110-A3 SIEM and 6110-A4 JUNIOR pump and accessories must be operated within a temperature range of +5 °C
and +40 °C (+41 °F and +104 °F). Do not operate the products in extreme cold or heat.
9.3 Transport/Storage/operating conditions (humidity)
The 6110-A3 SIEM and 6110-A4 JUNIOR pump and accessories must remain in the packaging for storage and used at a
humidity range from 15% to 93 %.
9.4 Transport/Storage/operating conditions (atmospheric pressure)
The 6110-A3 SIEM and 6110-A4 JUNIOR pump and accessories must remain in the packaging for storage and used at an
atmospheric pressure range from 0,7 bar to 1,06 bar
CAUTIONS: Do not operate the 6110-A3 SIEM and 6110-A4 JUNIOR pump above 3000 m above sea level
(9,84 ft).
IP33
9.5 Protection class
The 6110-A3 SIEM and 6110-A4 JUNIOR pump are protected against ingress of dripping water (IP33).
The 6110-A3 SIEM and 6110-A4 JUNIOR equipments are protected against the penetration of liquids and
solids (IP33) It is always good though protect from heavy rains. During operation and storage, the device should be
kept dry. If the device is entirely wet, move it to a dry area, dry externally and wait at least 30 minutes before using
it again if you are sure that water didn’t enter inside.
6110-A3 SIEM technical data
Dimensions (h x w x l): 965 x 400 x 330 mm (3 x 15,7 x 13 inches)
Voltage: 230 VAC; 50 Hz; 12 VA
Operating cycle: continuous
flow rate (before the filter): > 60 liters/minute (+/– 5 %)
Standard Canister volume: 2000 ml
weight (without canister): 13 kg / 2 lbs approx
max. vacuum level: – 652 mmHg / – 7 kPa /- 0, 7 bar (+/- 5 %) (*)
CEI EN60601-1 Classification: Insulation Class I device - Applied part type B
Device not suitable for use in presence of flammable anesthetic mixture with air,
oxygen or nitrogen protoxid.
UNI EN ISO10079-1 Classification: Equipments with HIGH VACUUM level and HIGH FLOW
DDM 93/42 CEE Classification: Class ll b

USER and MAINTENANCE MANUAL 6110 A3 and 6110 A4
Manufacturer: Siem Nova S.r.l. Legal and operational offices 200 7 ROZZANO (Milano) MADE IN ITALY
Product: SURGICAL ASPIRATOR MOD: 6110-A3 SIEM and 6110-A4 JUNIOR
Updated: 2018-03-19 reproduction forbidden Pag: 9 di 10
6110-A4 JUNIOR technical data
Dimensions (h x w x l): 222 x 320 x 360 mm ( ,5 x 12,5 x 14 inches)
Voltage: 230 VAC; 50 Hz; 12 VA
Operating cycle: continuous
flow rate (before the filter): > 60 liters/minute (+/– 5 %)
Standard Canister volume: 2000 ml
weight (without canister): 6 kg / 13 lbs approx
max. vacuum level: – 652 mmHg / – 7 kPa- 0, 7bar (+/- 5 %) (*)
CEI EN60601-1 Classification: Insulation Class I device - Applied part type B
Device not suitable for use in presence of flammable anesthetic mixture with air,
oxygen or nitrogen protoxid.
UNI EN ISO10079-1 Classification: Equipments with HIGH VACUUM level and HIGH FLOW
DDM 93/42 CEE Classification: Class ll b
6110-A4 JUNIOR WITH TROLLEY Technical data
Dimensions (h x w x l): 1030 x 640 x 640 mm (40,5 x 25 x 25 inches)
Voltage: 230 VAC; 50 Hz; 12 VA
Operating cycle: continuous
flow rate (before the filter): > 60 liters/minute (+/– 5 %)
Standard Canister volume: 2000 ml
weight (without canister): 10 kg / 22 lbs approx
max. vacuum level: – 652 mmHg / – 7 kPa- 0, 7bar (+/- 5 %) (*)
CEI EN60601-1 Classification: Insulation Class I device - Applied part type B
Device not suitable for use in presence of flameable anaesthetic mixture with air,
oxygen or nitrogen protoxid.
UNI EN ISO10079-1 Classification: Equipments with HIGH VACUUM level and HIGH FLOW
DDM 93/42 CEE Classification: Class ll b
(*) Measured at 0-meter, atmospheric pressure (1013.25 hPa).
PLEASE NOTE: vacuum levels may vary depending on location (meters above sea level, atmospheric
pressure and temperature).
10 DISPOSALS
At the end of their operative life the device and accessories must be disposed of in compliance with the local regulation and
environmental laws, if no legal regulation exists the different material must be sorted and disposed of separately (see the below
table)
Remark: the components of the equipments or accessories don’t contain phthalates or natural latex
item Material or regulation
Suction pump unit and power cord Waste from Electric and Electronic Equipment (WEEE)
Unit case NON-MAGNETIC ALUMINIUM frame
Jar Polycarbonate
Lid Polycarbonate
Rubber components Silicone
Cage and float Polypropilene
box Cardboard
manual Paper
11 ELECTROMAGNETIC COMPATIBILITY
The suction pump 6110-A3 SIEM and 6110-A4 JUNIOR are supplied with an electric induction motor and has no electronic parts so
for his own nature he doesn´t generate electromagnetic emissions or is influenced by external electromagnetic emissions, this
means that this device is automatically in compliance with the requirements of CEI EN 60601-1-2-2015 for the electromagnetic
compatibility of medical devices, anyhow the use of mobile telephones, LAN / WLAN, walkie-talkies (two-way radios) and cordless
telephones sets could affect the 6110-A3 SIEM and 6110-A4 JUNIOR pump. A safety distance of min. 3.3 ft (1 m) to the
equipment is recommended.
RECOMMENDED SEPARATION DISTANCES BETWEEN RADIOCOMMUNICATION DEVICES
The surgical suction equipments 6110-A3 SIEM and 6110-A4 JUNIOR Don´t generate electromagnetic noises and are not
influenced by external electromagnetic noises, anyhow it is better to use it in an electromagnetic environment in which radiated
RF disturbances are under control. The customer or the 6110-A3 SIEM and 6110-A4 JUNIOR operator of the device can help
prevent electromagnetic interference by maintaining a minimum distance between mobile and portable RF communication
equipment (transmitters) and the 6110-A3 SIEM and 6110-A4 JUNIOR device as recommended below, in relation to the
maximum output power of the communications equipment.
USER and MAINTENANCE MANUAL 6110 A3 and 6110 A4
Manufacturer: Siem Nova S.r.l. Legal and operational offices 200 7 ROZZANO (Milano) MADE IN ITALY
Product: SURGICAL ASPIRATOR MOD: 6110-A3 SIEM and 6110-A4 JUNIOR
Updated: 2018-03-19 reproduction forbidden Pag: 10 di 10
Maximum nominal power of the
transmitter output (W)
Separation distance to the frequency of the transmitter (m)
From 150 kHz to 0 MHz
d=1,2 x √P
from 0 kHz to 00 MHz
d=1,2 x √P
From 00 kHz to 2,5 GHz
d=2,3 x √P
0,01 0.12 0.12 0.23
0,1 0.3 0.3 0.73
1 1.2 1.2 2.3
10 3. 3. 7.3
100 12 12 23
For transmitters with a maximum rated output power not listed above, the recommended separation distance "d", in meters
(m) can be calculated using the equation applicable to the frequency of the transmitter, where "p" is the maximum output
power of the transmitter in watts (W) according to the transmitter manufacturer.
Note 1: At 0 MHz and 00 MHz, the exposure distance for the higher frequency range is applicable.
Note 2: These guidelines may not apply to all situations. Electromagnetic propagation is affected by absorption and reflection
from structures, objects and people.
1 SPARE PARTS
Use only accessories or spare parts supplied by Siem Nova Srl. The use of spare parts not supplied from
Siem Nova Srl could invalidate the warranty.
13 WARRANTIES
Siem Nova srl (or his authorized distributor) warrants that the device will be free from defects in materials
and workmanship for a period of years from the date of delivery.
Faulty material will be replaced free of charge during this period if not resulting from abuse or misapplication.
Transport costs are not included. The warranty includes defects in materials, components and/or workmanship only
if:
A. THE DEVICES ARE USED IN RESPECT TO ALL INSTRUCTIONS OF OPERATOR’S MANUAL;
B. MAINTENANCE IS DONE BY SIEM-NOVA SRL AUTHORIZED PERSONNEL;
C. THE ACCESSORIES ARE SUPPLIED BY SIEM NOVA
This will not apply to parts subject to wear and tear in use (i.e: filters, power cord, lid and canisters).
To better ensure compliance with this warranty we recommend the exclusive use of spare parts supplied by Siem
Nova srl.
The right to the replacement of faulty parts will not be recognized by Siem Nova srl if any work has been made on
the equipments by unauthorized persons.
14 Technical features update
In order to continuously improve performance, safety and reliability, all products medical devices from Siem
Nova Srl are periodically reviewed and changed. The instruction manuals are therefore amended to ensure their
continued compliance with the characteristics of the input devices on the market. If the instruction manual
accompanying this device is lost, you can obtain from the manufacturer a copy of the version corresponding to the
device provided supplying serial number on the device label.
This manual suits for next models
3
Table of contents
Other Siem Nova Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Concoa
Concoa Medical IntelliSwitch 570 Series Installation and operating instructions
B.A. International
B.A. International Optima EOS350 Instructions for use and installation

Orliman
Orliman JEWETT J001G manual

laerdal
laerdal VitalsBridge Directions for use

iMediSync
iMediSync iSyncWave Instructions for use

eitan medical
eitan medical Sapphire quick guide