#65060/INTL-K-10.2020 MORIA 2
This instruction manual is for EVOLUTION 3E with serial numbers 5000 and above.
For the EVOLUTION 3E with serial numbers below this number, please refer to user manual (#65051).
The most recent version of this user guide and additional information on your keratome are available on
MORIA website: http://www.moria-surgical.com.
I. DISCLAIMER
A. MAINTENANCE AND WARRANTY
The EVOLUTION 3E system has been designed for optimal operation, provided that the recommendations
listed in this user manual are followed carefully.
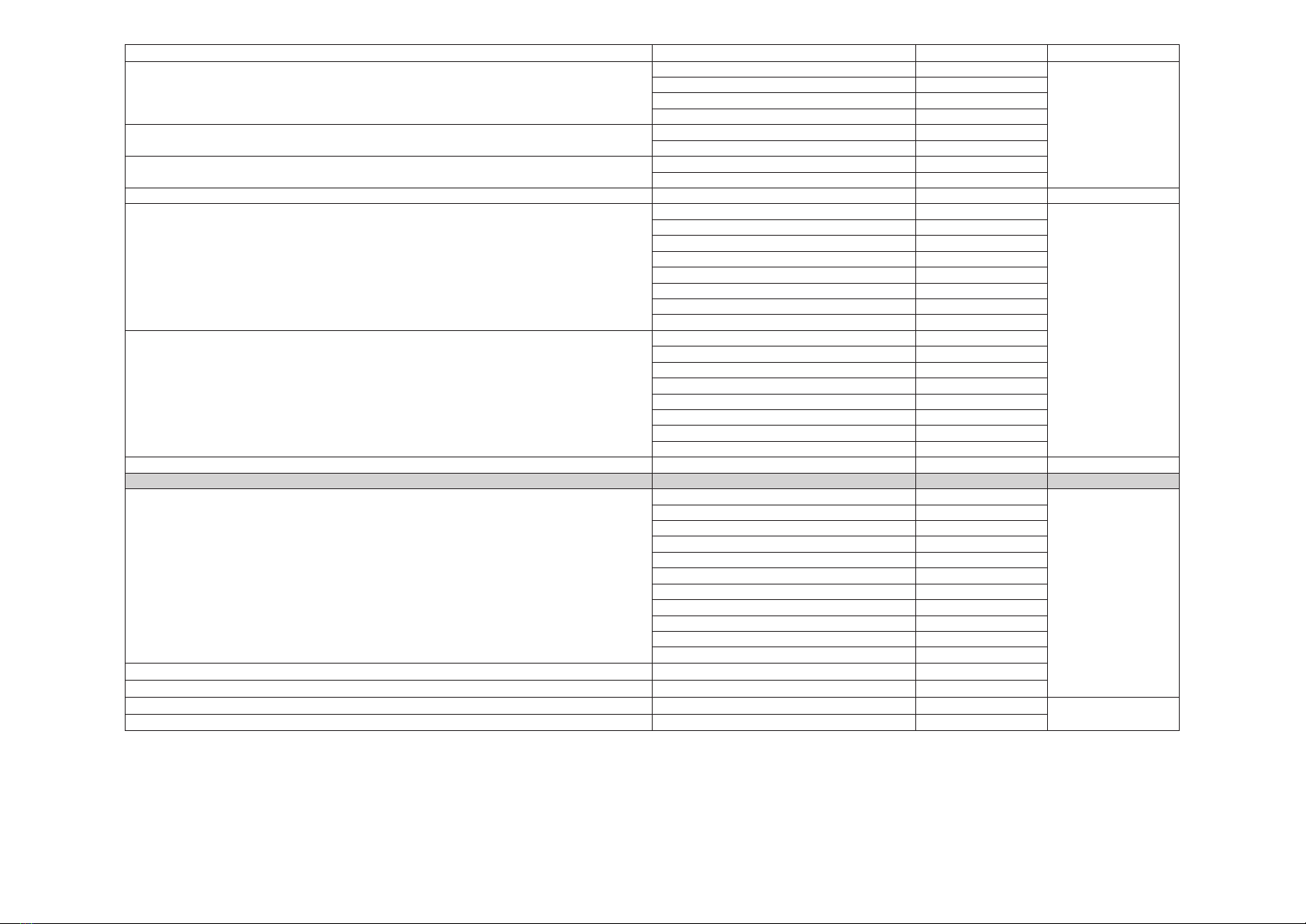
The lifetime of the Evolution 3E system is 5 years. This lifetime can be extended to 15 years if the maintenance
plan described above is strictly followed. The maintenance operations in the fth and tenth years (M5 and
M10) must be carried out by Moria. They guarantee the electrical safety and operating performance of the
Evolution 3E Console and thus the user’s and the patient’s safety.
The other annual, intermediate maintenance operations will be carried out by Moria or by its representative
who will have been previously trained and authorized by Moria. This representative must have a valid training
certicate and must use only spare parts supplied by Moria.
MORIA shall not guarantee the performance of the Evolution 3e system and patient and user safety if the
maintenance plan is not followed.
Years Ref. Check Electrical
safety
Remplacement
of the primary
circuit
Battery
change
Turbine
gasket
change
Pump
change
Tubing
change
Engine board
change
Vacuum
solenoid
valve change
1M1 yes no no no yes no yes no no
2M2 yes no no no yes no no no no
3M3 yes no no no yes no yes no no
4M4 yes no no no yes no no no no
5 M5 yes yes no yes yes yes yes no no
6M6 yes no no no yes no no no no
7M7 yes no no no yes no yes no no
8M8 yes no no no yes no no no no
9M9 yes no no no yes no yes no no
10 M10 yes yes yes yes yes yes yes yes yes
11 M11 yes no no no yes no no no no
12 M12 yes no no no yes no yes no no
13 M13 yes no no no yes no no no no
14 M14 yes no no no yes no yes no no
15 No maintenance
If, for any reasons, the system does not perform properly, have it checked immediately by MORIA. MORIA
strongly recommends having the system thoroughly inspected by MORIA on a routine basis every year.
The use of materials and/or components of a brand other than MORIA with the EVOLUTION 3E system will
immediately nullify the MORIA warranty. MORIA may not be held responsible for any damage resulting from
the use of materials and/or components of a brand other than MORIA.
As only MORIA and its agents are fully expert in MORIA products, servicing and maintenance must be carried
out by MORIA or its approved agents.
MORIA shall not be held liable for any malfunction or damage to the apparatus, poor results, or surgical
complications due to maintenance being having been carried out by an unqualied operator or third party.
Any such unauthorised intervention shall render the guarantee and any maintenance contract null and void.
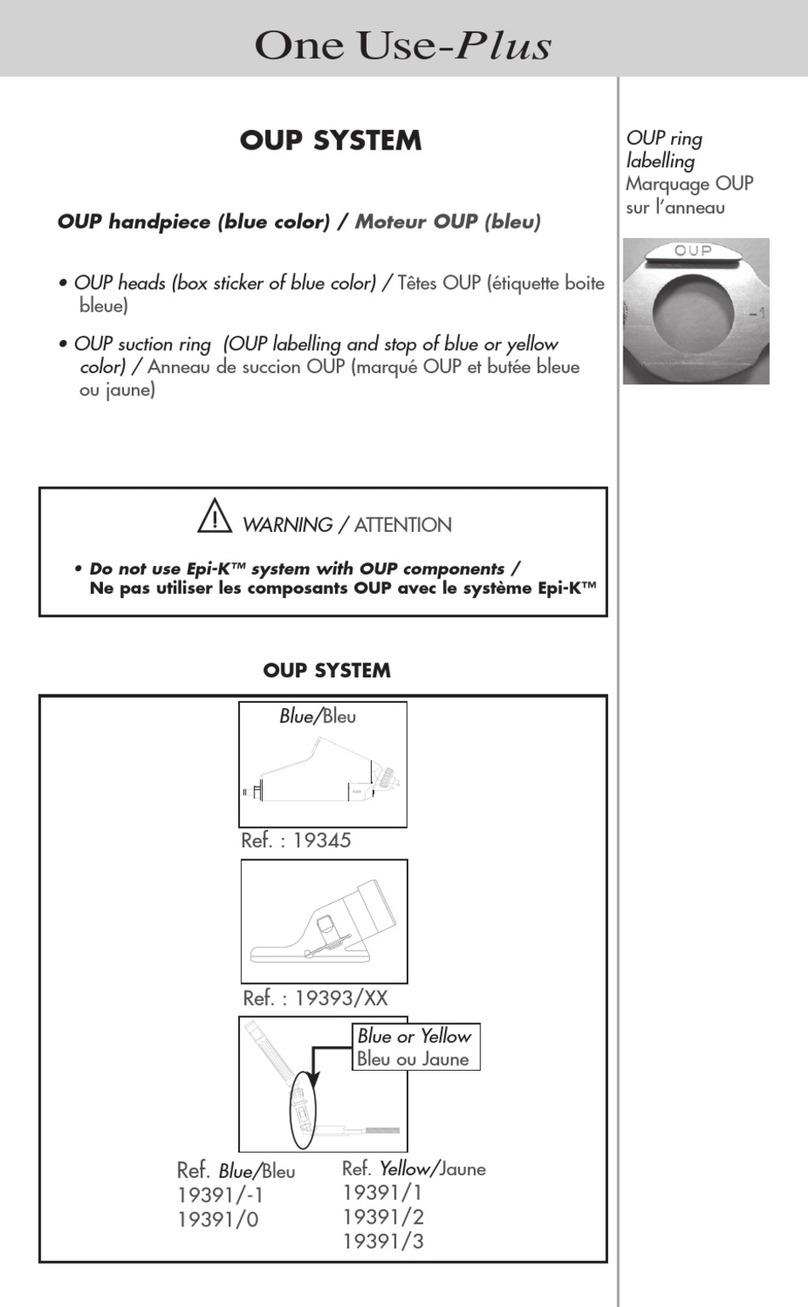
B. USE OF GENERIC PRODUCTS AND REUSE OF SINGLE-USE CONSUMABLES
Single-use devices should not be re-used. Doing so will negatively affect their clinical performance and
increase the potential for adverse events.
The reuse of single-use products, or the use of consumables other than those supplied by MORIA, may entail
serious surgical consequences for the patient and dama ge the microkeratome.
MORIA shall not be held liable in the event of a malfunction or damage to the microkeratome, poor results or
surgical complications due to the reuse of a single-use product, or the use of consumables other than those
supplied by MORIA.
MORIA handpieces must only be connected to MORIA devices (console unit, heads, suction rings, etc.).
All warranties become null and void if the microkeratome degrades or malfunctions due to such practices.
The most recent version of this user guide and additional information on your keratome are available on
MORIA website: http://www.moria-surgical.com.
II. GUIDANCE AND MANUFACTURER’S DECLARATION: ELECTROMAGNETIC
EMISSIONS AND IMMUNITY
Refer to annexe document (#65073).
III. EQUIPMENT AND ACCESSORIES LIST
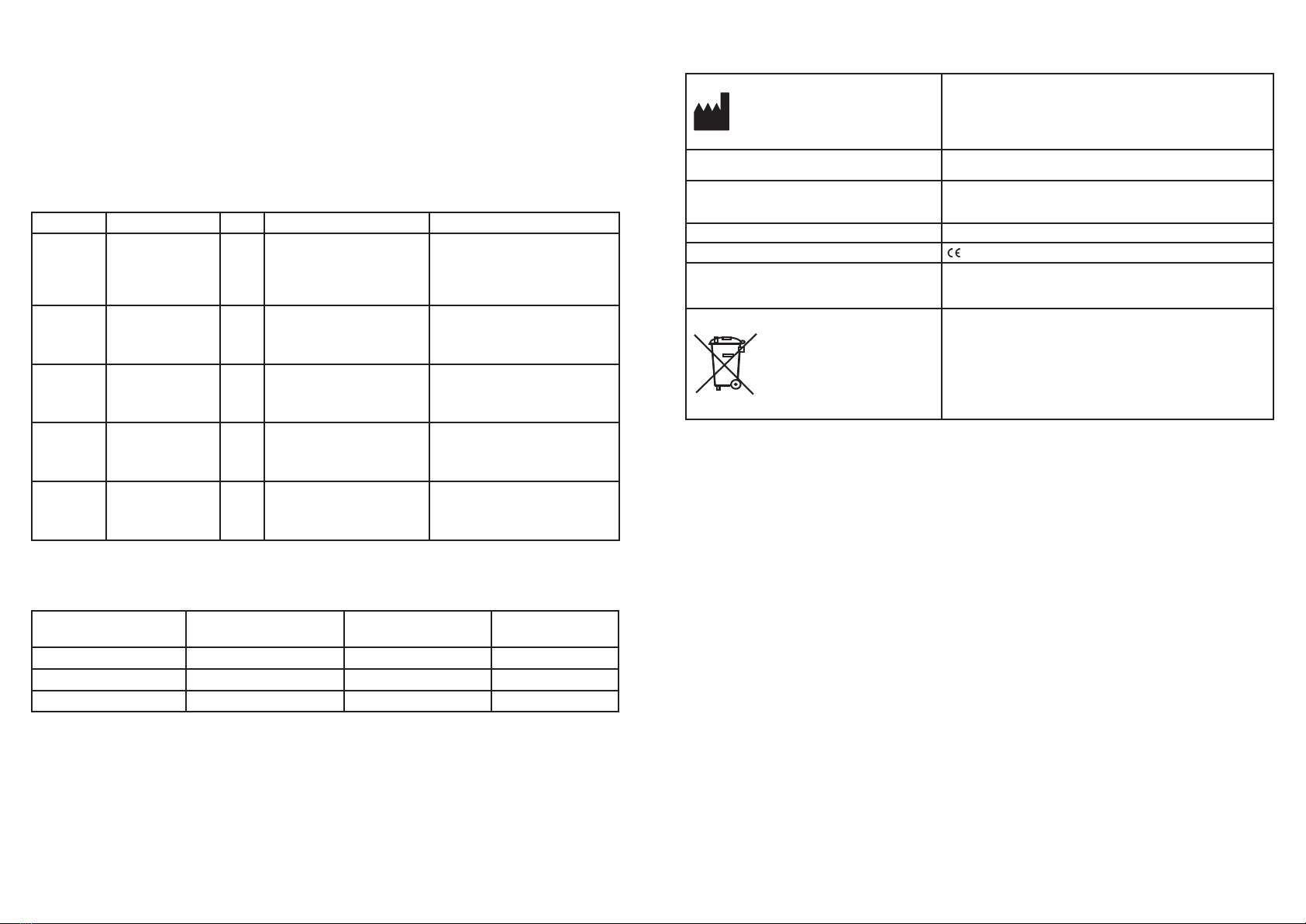
A. EQUIPMENT LIST
Designation Regulatory
designation
MORIA
reference
EVOLUTION 3E Console (S/N above 5000) EVOLUTION 3e CONTROL
UNIT
19380
EVOLUTION 3-3E Control Footpedal FOOT PEDAL FOR
EVOLUTION
19361
EVOLUTION 3E Control Footpedal Epi-K™ EVOLUTION 3e
FOOTSWITCH
19381
Evolution 3E Footswitch - China EVOLUTION 3e
FOOTSWITCH
19381C
Evolution 3E footswith - Japan EVOLUTION 3e
FOOTSWITCH
19381J
EVOLUTION 3 Supply Cords (CEE) (2.50m) / Cable (CEE) EVOLUTION 3 SUPPLY
CORD (EEC)
19362
EVOLUTION 3 Supply Cords (USA) (2.50m) / Cable (USA) EVOLUTION SUPPLY
CORD (USA)
19363
EVOLUTION 3 Supply Cords (UK) (2.50m) / Cable (UK) EVOLUTION 3 SUPPLY
CORD (UK)
19364
EVOLUTION 3 Supply Cords (China) (2.50m) / Cable (China) EVOLUTION 3 SUPPLY
CORD CHINA
19516
EVOLUTION 3 Supply Cords (Brazil) (2.50m) / Cable (Brazil) EVOLUTION 3 SUPPLY
CORD BRAZIL
19521
Supply cord USA SUPPLY CORD (USA) 19451
Carrying Case N/A 19511
User manual N/A 65060/INTL
User manual - China N/A 65060/ZH
User manual- Brazil N/A 65060/BR
Annexe “Guidance and manufacturer’s declaration: electromagnetic emissions and
immunity”
N/A 65073