Signifier eXciteOSA User manual

Physician Guide
IMPORTANT SAFEGUARDS:
READ ALL INSTRUCTIONS BEFORE USE

Contents
Important safeguards ................................................................................................................ 3
Intended use............................................................................................................................... 5
Contraindications....................................................................................................................... 6
What’s in the box? ...................................................................................................................... 7
eXciteOSA® smartphone application......................................................................................... 9
Using your eXciteOSA® with a smartphone application ......................................................... 13
My eXciteOSA® phone application ........................................................................................... 15
Frequency of usage .................................................................................................................. 20
Charging the Control Unit........................................................................................................ 20
Storing & traveling with your eXciteOSA®................................................................................ 21
Washing .................................................................................................................................... 21
Soware updates ..................................................................................................................... 21
Settings..................................................................................................................................... 22
Frequently asked questions..................................................................................................... 24
Troubleshooting....................................................................................................................... 26
Cybersecurity............................................................................................................................ 27
eXciteOSA® system, operational requirements....................................................................... 29
eXciteOSA® system, transport and storage requirements ...................................................... 29
Symbols used ........................................................................................................................... 31
Specications........................................................................................................................... 32
Declarations.............................................................................................................................. 35
Table A: Representative output with varying loads................................................................ 37
3
Important safeguards
WARNINGS
To avoid the risk of electrocution, burns, re or physical injury:
• The device is only to be used while awake. It should not be used while sleeping.
• Only the supplied products should be used together. Do not use attachments other than
those recommended by the manufacturer.
• Discontinue use if the product appears damaged in any way.
• Never attach the Mouthpiece directly to a charging source other than the control unit provided.
• This device should only be charged using the USB Cable provided.
• Do not operate in close proximity (e,g. 3.4 feet) to a shortwave or microwave unit as other
Electromagnetic equipment may produce instability in the simulator output.
• Always keep the Control Unit away from water.
• Unplug the Control Unit immediately if it falls into water.
• Never use a damaged USB Cable to charge the Control Unit.
• Recharge the battery using only the USB power cord with a UL-certied USB wall charger
(not provided).
• This product contains no user serviceable parts. Refer to “Warranty” and
“Troubleshooting” if appliance no longer works as expected.
• The device is only to be used as specied in this document.
CAUTION:
FEDERAL LAW RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN
READ ALL INSTRUCTIONS BEFORE USE.
(for latest User Guide, please refer to https://exciteosa.com)
4
WARNINGS
To avoid the risk of physical injury:
• Do not use this device if you are under the age of 18
• eXciteOSA® should be kept away from children and pets
(choking hazard and electrical hazard).
• eXciteOSA® to be used in a home or healthcare environment (or equivalent).
• eXciteOSA® is a single person device and should not be shared or used by other
individuals.
• Do not use eXciteOSA® when driving a vehicle or operating machinery and other
equipment.
• When charging the device, keep away from all liquids.
• No modication of this device is allowed. Do not try to alter, trim or change any
components of the device.
• The silicone on the Mouthpiece must be intact before use. If there is any breach of the
silicone (including electrodes) - DO NOT USE THE MOUTHPIECE.
READ ALL INSTRUCTIONS BEFORE USE.

5
Intended use
eXciteOSA® is intended for the reduction of snoring and mild obstructive sleep apnea by
strengthening tongue muscles via electrical muscle stimulation.
INDICATIONS FOR USE
eXciteOSA® is a removable tongue muscle stimulation device that delivers neuromuscular
stimulation to the tongue in order to reduce mild obstructive sleep apnea (AHI <15) and
snoring for patients that are 18 years or older.
ADDITIONAL CLINICIAN AND PATIENT INSTRUCTIONS FOR USE
• Patients should have a comprehensive dental examination prior to using this device to
rule out cavities, dental implants, metal prosthesis, metal braces, dental appliances, and
intraoral metallic jewelry/piercings.
• Patients should maintain regular follow-up visits with a Dentist and Sleep Health
Professional.
• Patients should follow up with their sleep health clinician for a repeat Home Sleep Apnea
Test aer three months of therapy.
CLINICAL SUMMARY
• eXciteOSA® was used by 65 mild OSA patients, 20 minutes per day for 6 weeks, and 79%
of group (51 patients) achieved an average reduction of 52% in AHI.
• eXciteOSA® was used by 115 snoring patients, 20 minutes per day for 6 weeks, and 90% of
the group achieved an average reduction of 46% of time spent snoring.

6
CONTRAINDICATIONS
Do not Use eXciteOSA® if you:
• are pregnant or may be pregnant.
• have a pacemaker or implanted
electrodes.
• have temporary or permanent metal
implants, dental braces, intraoral metal
prosthesis/restorations/appliances or
dental jewelry in the mouth.
• are suffering from mouth ulcers.
• have or are suspected of having an AHI
≥ 15 as determined by evaluation by a
Sleep Health Professional with a sleep
study.
PRECAUTIONS
Consult your Doctor/Dentist if you:
• have gum disease or have bleeding from
your gums or other oral conditions.
• experience pain, numbness or bleeding
aer using this product.
• have any medical concerns.
This device should not be used:
• while asleep (the device should only be
used when awake).
• in contact with your head, neck, spine,
chest, eyes, ears or any other parts of
the body other than inside the mouth as
instructed.
• the device has not been tested for MRI
compatibility and should not be used in
the vicinity of an MRI device.
Adverse Reactions:
• accumulation of saliva.
• tingling sensation on tongue.
• tooth sensitivity.
READ ALL INSTRUCTIONS BEFORE USE.
Safety and effectiveness in the
above conditions have not been
established.

7
Mouthpiece (Top view)
Electrodes
Flexible silicone
USB-C type connector
Fig.1
What’s in the box?
eXciteOSA® package includes:
1

8
2
3
Control Unit (With Bluetooth)
USB Cable (15 cm cable connect to UL-Certied USB wall
charger (not provided) with 5 VDC, Min 0.5 AMP output)
USB-C type connector
LED light under the logo
Fig.2
Fig.3

9
Systems requirements
eXciteOSA® smartphone application
The eXciteOSA® device can be controlled by the smartphone application. This app can be
downloaded from the App store (Apple iOS) or Play Store (Google Android)
The mobile app soware can be used on iPhone 5S, iPhone 6/6 Plus, iPhone 6s/6s Plus,
iPhone 7/ 7 Plus, iPhone 8/8 Plus, iPhone X, with iOS 11.0 and higher. The mobile app
soware can also be used with Android devices with BLE support (Bluetooth 4.0) and
Android 7.0 and above. eXciteOSA® uses Bluetooth Smart; mobile devices used must be
compatible with Bluetooth Smart.
eXciteOSA® device can be controlled by eXciteOSA® Smartphone
application. Smartphone application can be downloaded from
App Store (Apple iOS) or Play Store (Google Android).
The log in & sign up section allows you to
authenticate or create an account.
Enter the e-mail you want to register with
Enter a secure password (minimum 6 characters)
If you are already registered you can log in here
You can sign up with one of these services
(this will require authentication on the appropriate external service)
LOG IN & SIGN UP
SIGN UP
iPhone, iTunes, and iOS are registered trademarks of Apple, Inc.
Bluetooth is a registered trademark of Bluetooth SIG, Inc.

10
Enter your full name
Enter your country of residence
Select your gender
Select your date of birth
Enter your ethnicity, height and weight*
* This data allows for better eXciteOSA therapy options. Your data
is secure with us. You can check our privacy policy, on our website
https://exciteosa.com/privacy-policy, for further details.
Select whether you already have
a eXciteOSA® device or not.
If you choose yes, additional setup is required.
PROFILE SET UP
11
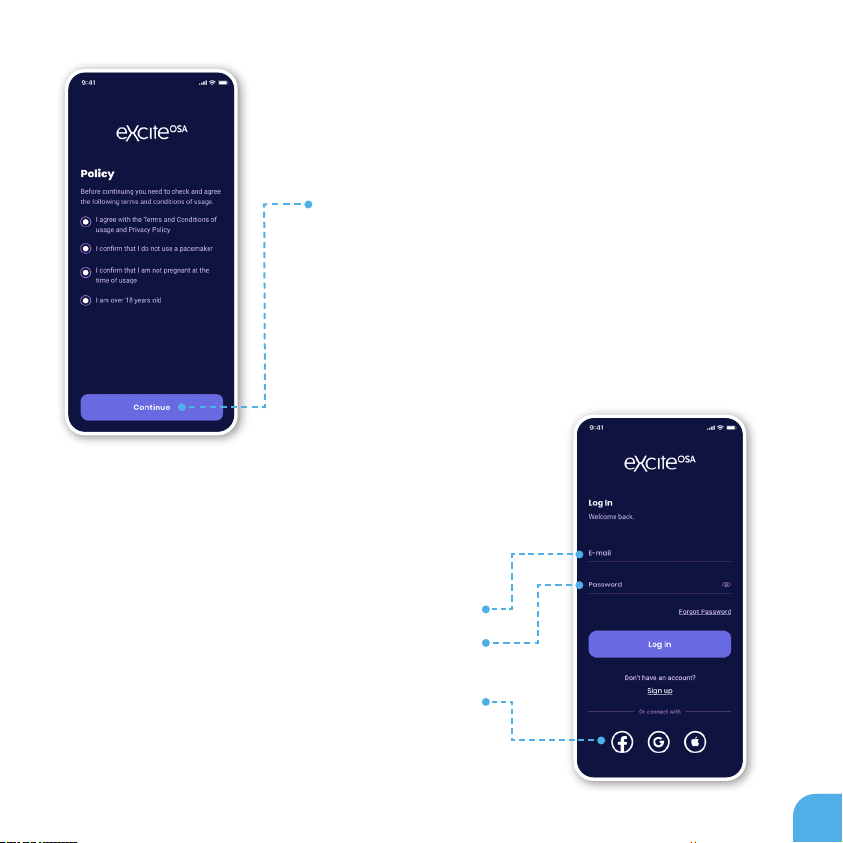
There are a number of terms you have to agree
to in order to use the eXciteOSA® device with the
mobile app.
First, make sure you read the Terms and Conditions
page, and agree with them.
Next, please conrm that you do not use a
pacemaker and that you are not pregnant. It is
very important that none of these apply to you
as the eXciteOSA® device can interfere with your
pacemaker or with your baby’s development.
Also, you must be 18 years of age or older.
POLICY
Enter your registered e-mail and password
If you forgot your password, tap here. You will
receive an e-mail with further instructions.
You can log in with one of these services
(this will require authentication on the appropriate external service)
LOG IN

12
The dashboard is the main screen of the app where all the features are available from.
THE DASHBOARD
Settings
eXciteOSA® therapy
Educational content

13
Using your eXciteOSA® with a
smartphone application
Before starting your therapy be sure to: check the silicone on the Mouthpiece is not cracked
or broken before use. If there is any evidence of damage to the silicon - DO NOT USE THE
MOUTHPIECE.
Before use, wash the Mouthpiece with cold and running tap water as shown in Fig 4. Avoid
water reaching the USB port. Dry with clean towel.
Fig.4

14
To start using eXciteOSA®, connect the
Mouthpiece with the Control Unit as
shown on Fig 5.
Insert the Mouthpiece into the
mouth and allow it to sit around the
tongue as shown in Fig 6. and gently
close your mouth. Avoid grinding or
clenching the Mouthpiece.
Fig.5
Figure 6

15
My eXciteOSA® phone application
My eXciteOSA® is the place where you can start your eXciteOSA® therapy or add your device.
This screen will appear if you haven’t already
added a device.
Before starting your therapy be sure to: check
your device for cracks or broken parts, connect
the controller to the mouthpiece, insert the
mouthpiece into the mouth.
Add a eXciteOSA® device
(make sure that the device is nearby)
In order for the eXciteOSA® device to work,
you need to allow Bluetooth connection.
For iOS 13 or above you need to manually
allow Bluetooth permission.

16
Recommended Therapy Level
The patient is recommended to set the device to the highest possible therapy level that
is still comfortable to them. This will ensure the best possible outcome. Tolerance levels
are likely to increase each week, and patients are encouraged to continue to raise levels
accordingly. Levels that feel painful or uncomfortable will not speed up outcome or
improve results.
Each 20 session is broken up into four 5-minute intervals. Each interval will prompt the
device to give off a different frequency. The frequencies will not exceed 20 Hz.
Therapy Level Guide
Therapy levels can be changed using the arrow buttons on the app.
THERAPY LEVEL RANGES
POTENTIAL THERAPY
LEVEL PATHS WEEK 1 WEEK 2 WEEK 3 WEEK 4 WEEK 5 WEEK 6
FOR A LOWER
TOLERANCE PATIENT 1 TO 3 3 TO 4 4 TO 5 4 TO 5 5 TO 6 6 TO 7
FOR A MEDIUM
TOLERANCE PATIENT 3 TO 5 6 TO 8 9 TO 10 11 TO 12 11 TO 12 12 TO 13
FOR A HIGHER
TOLERANCE PATIENT 4 TO 7 8 TO 9 10 TO 12 13 TO 14 14 TO 15 14 TO 15
Please note that these are approximations. Every patient is likely to have a different
tolerance level. This example chart should serve as a suggestion that can be used to help
guide your patient.
17
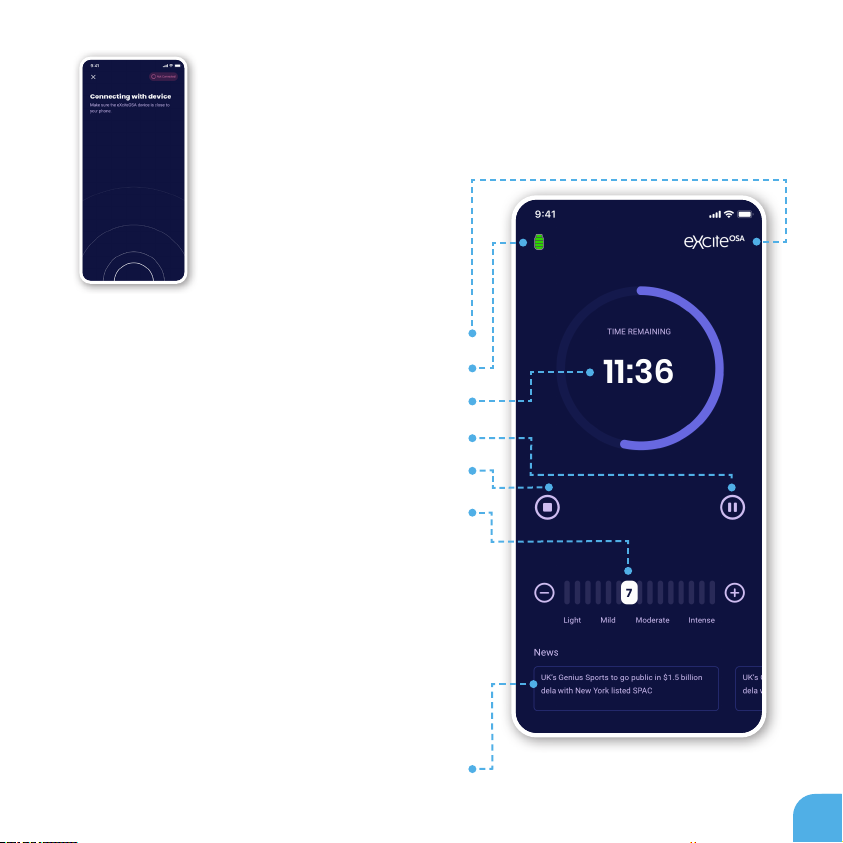
In order to start the therapy, you need to pair your phone with the control
unit. Please make sure that the control unit is close to your phone.
CONNECT
APPLICATION DURING
A THERAPY SESSION
Controlling your therapy level
Indicates if the device is connected to the phone
Indicates the device battery status
Indicates the remaining time of the therapy
Pauses the therapy
Stops the therapy
To change your therapy level, simply slide the level
setting at the bottom of your screen during your session.
It is important to set the device to the highest possible
therapy level that still feels comfortable for you. This will
ensure the best possible outcome.
If therapy becomes painful or uncomfortable, simply
remove the mouthpiece or lower the therapy level.
Please follow the advice from the prescribing clinician on
recommended starting therapy level.
Reading mode/Newsletter
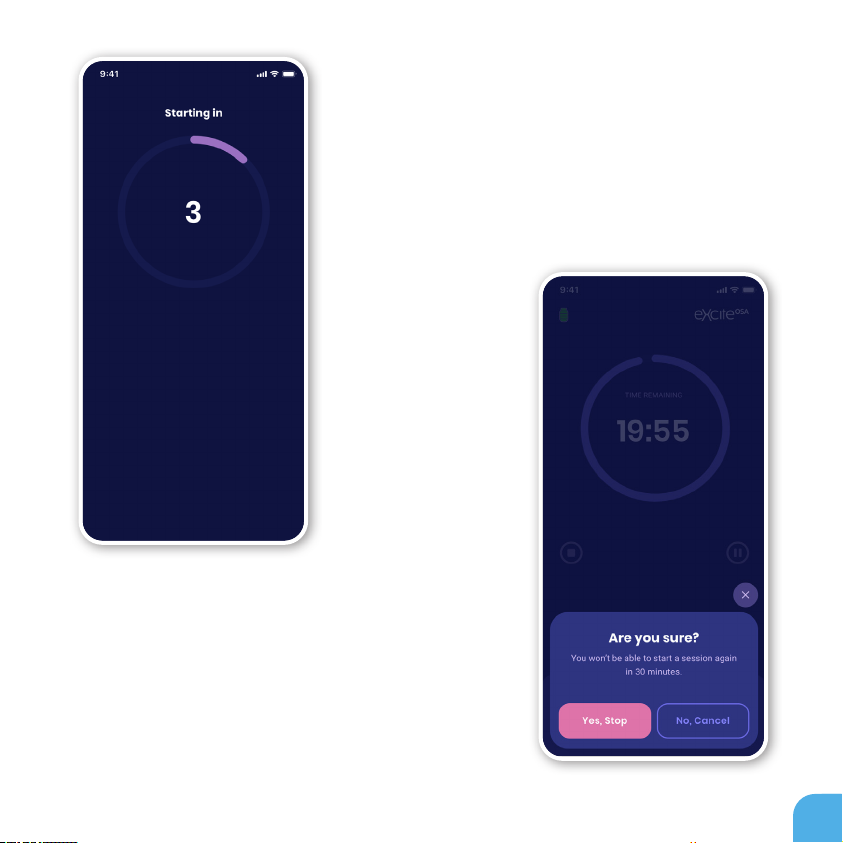
18
Aer pairing your eXciteOSA® device with your mobile
phone, your therapy will start with a countdown from
3 to 1 before initiating the actual therapy.
Therapy can be stopped at any time but due to safety
reasons it can only be restarted aer 30 minutes aer
the device has been stopped or the therapy has ended.
Keep this in mind when stopping the therapy.
COUNTDOWN
STOP THERAPY

19
Therapy can be paused at any time. It can be paused
only for 3 minutes. Aer the 3 minutes have passed
the therapy is considered stopped and can only be
restarted aer 30 minutes.
Therapy can be paused at any time. It can be paused only for 3 minutes. Aer the
3 minutes have passed the therapy is considered stopped and can only be restarted
aer 30 minutes.
You can set a reminder at a specic time each
day, so that you don’t miss your therapy.
PAUSE THERAPY
END OF THERAPY SESSION
SET A REMINDER
20
Frequency of usage
It is recommended you use eXciteOSA® once daily for a continuous 20 minutes. It can take
up to 6 weeks of daily therapy before you will gain the desired improvement in your mild
OSA or snoring. Aer 6 weeks of therapy, only two, 20-minute sessions are required each
week thereaer. eXciteOSA® can be used long term. This is comparable to maintaining your
physical tness by undertaking regular exercise.
You must replace the Mouthpiece aer three months of rst use, which includes the
maintenance period.
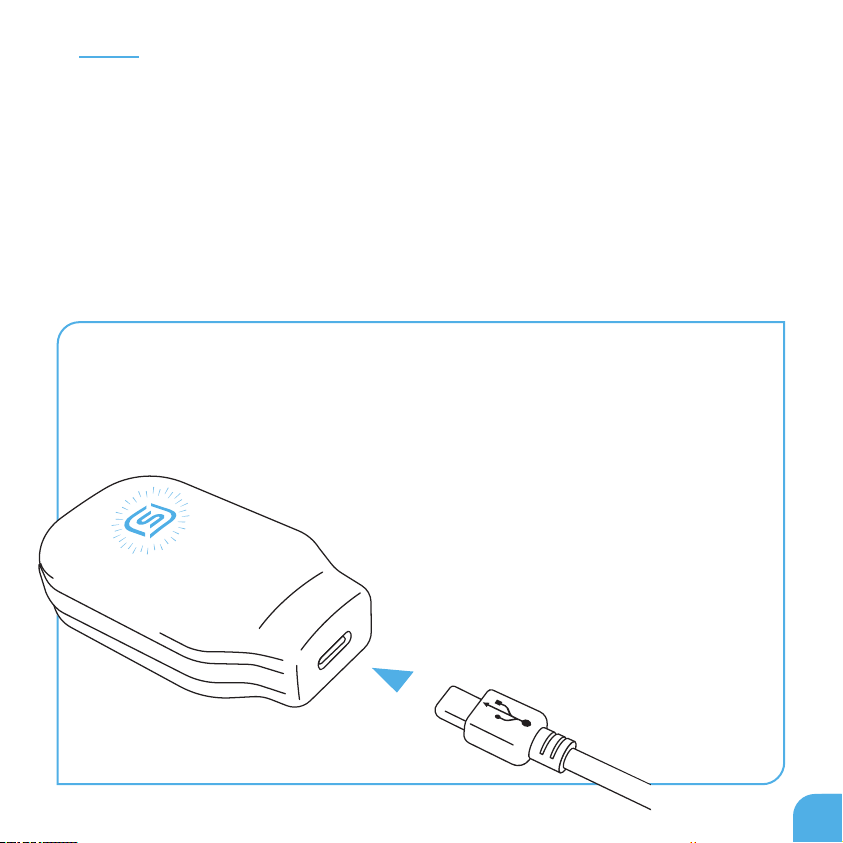
Charging the control unit
Before using the device for the rst time, you must
charge the battery for at least 2 hours. The Control Unit
LED will ash blue and amber if there is inadequate
charge to run a full 20 minute therapy session. When
Control Unit logo (Fig 8) blinks blue and amber, recharge
the Control Unit. To charge the Control Unit, use
the USB Cable provided and connect the Control
Unit jack into a USB power adapter. During
charging, the eXciteOSA® logo will blink.
When fully charged, the eXciteOSA® logo
will become continuous blue.
Fig.8
Other manuals for eXciteOSA
1
Table of contents
Other Signifier Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual












