Simex cuff M User manual

GA_CuffM-S_3rd_EN_2016-06-09_2017-04-11_Rev.C00_USA
AS TO ELECTRICAL
SHOCK, FIRE AND
MECHANICAL HAZARDS
ONLY IN ACCORDANCE
WITH ANSI/AAMI
ES60601-1 (2005),
CAN/CSA-C22.2 No.
60601-1 (2008)
Instruction for Use
simex cuff M and simex cuff S
Automated Subglottic Aspiration System
0843

page 2 of 36
Copyright © 2016 simex Medizintechnik GmbH, Deisslingen.
The safety of the simex cuff Mand simex cuff S complies with the acknowledged rules of
technology and meets the requirements of the German Medical Devices Act.
The simex cuff Mand simex cuff S bear the CE marking CE0843 in accordance with EU
Council Directive 93/42/EEC concerning medical devices and meet the essential requirements of
Annex I of this directive.
The simex cuff M and simex cuff Shave been tested in accordance with IEC 62353.
The quality management system applied by simex Medizintechnik GmbH is certified in
compliance with the relevant international standards.
The simex cuff Mand simex cuff Sare medical aspiration devices classified as class IIa
in accordance with EU Council Directive 93/42/EEC, Annex IX.
Errors and omissions excepted.
Federal Law restricts this device to sale or
rental by or on the order of a physician

page 3 of 36
Table of Contents
1 User Information ..................................................................................................... 5
1.1 Using this Instruction Manual ................................................................................ 5
1.2 Icons .................................................................................................................. 5
1.2.1 General Symbols ............................................................................................. 5
1.2.2 Device and Packaging ...................................................................................... 5
1.2.3 Display ........................................................................................................... 6
1.3 Symbol Convention .............................................................................................. 6
1.4 Glossary ............................................................................................................. 6
1.5 Intended Use ....................................................................................................... 7
1.5.1 Essential Features ............................................................................................ 7
1.5.2 Indications ...................................................................................................... 7
1.5.3 Contraindications ............................................................................................. 7
1.5.4 Restrictions on use .......................................................................................... 7
1.6 Basic Safety Instructions ....................................................................................... 8
1.7 User Requirements ............................................................................................... 9
1.8 Information on Product Liability ............................................................................. 9
1.9 Material Compatibility ........................................................................................... 9
2 Product Description ............................................................................................... 10
2.1 Whole View ........................................................................................................ 10
2.1.1 simex cuff M .................................................................................................. 10
2.1.2 simex cuff M product contents .......................................................................... 10
2.1.3 simex cuff S ................................................................................................... 11
2.1.4 simex cuff S product contents .......................................................................... 11
2.2 Product Properties ............................................................................................... 12
2.2.1 Disposable secretion canister for simex cuff M .................................................... 12
2.2.2 Information on the simex filter system for the simex cuff M ................................. 12
2.2.3 Information on the carbon filter of the simex cuff M ............................................ 13
2.2.4 Disposable secretion canister system for simex cuff S ......................................... 13
2.2.5 Information on the double filter system for simex cuff S ...................................... 13
2.2.6 Battery .......................................................................................................... 14
2.2.7 Pressure settings ............................................................................................ 14
2.3 Warranty ........................................................................................................... 14
3 Operation .............................................................................................................. 15
3.1 Set-Up and Startup ............................................................................................. 15
3.1.1 Startup ......................................................................................................... 15
3.1.2 Connecting the simex cuff M and simex cuff S .................................................... 16
3.1.3 Positioning of the simex cuff M ......................................................................... 16
3.1.4 Connecting the disposable secretion canister (250 ml) of the simex cuff M ............. 17
3.1.5 Positioning of the simex cuff S ......................................................................... 17
3.1.6 Connecting the simex disposable secretion canister system of the simex cuff S ...... 17
3.1.7 Connecting endotracheal and tracheal tubes with integrated suction port .............. 19
3.2 Operation of the simex cuff M and simex cuff S ...................................................... 19
3.2.1 Setting the run and pause time ........................................................................ 20
3.2.2 Language selection ......................................................................................... 21
3.3 Patient Mode ...................................................................................................... 22
3.4 Canister Replacement ......................................................................................... 23
3.4.1 Replacement of the disposable canister (250 ml) of the simex cuff M .................... 23
3.4.2 Replacement of the disposable liner (1,000 ml) of the simex cuff S ....................... 23
4 Maintenance .......................................................................................................... 24
4.1 Cleaning and Care ............................................................................................... 24
4.1.1 General Information ........................................................................................ 24
4.1.2 Cleaning and disinfection of the surface of the device .......................................... 24
4.1.3 Disposal of the disposable secretion canister for simex cuff M .............................. 25
4.1.4 Disposal of the disposable liner and the suction tube for simex cuff S .................... 25
4.1.5 Cleaning / disinfection of the external canister for simex cuff S ............................ 25
4.1.6 Cleaning / disinfection of the tubing accessories for simex cuff S .......................... 25
4.2 Maintenance and Service ..................................................................................... 25
4.3 Testing of the simex cuff M or simex cuff S ............................................................ 26
5 Problem Solving .................................................................................................... 26
5.1 Function Test ...................................................................................................... 26
5.2 Troubleshooting .................................................................................................. 26
5.3 Error Messages ................................................................................................... 27
6 Transport, Storage and Disposal............................................................................ 28
6.1 Decontamination prior to Shipment ....................................................................... 28

page 4 of 36
6.2 Storage ............................................................................................................. 28
6.3 Disposal ............................................................................................................. 28
7 Technical Data ....................................................................................................... 29
7.1 simex cuff M ....................................................................................................... 29
7.2 simex cuff S ....................................................................................................... 30
7.3 EMC Information ................................................................................................. 31
8 Ordering Information ............................................................................................ 35
8.1 simex cuff M ....................................................................................................... 35
8.2 simex cuff S ....................................................................................................... 35
9 Publishing Information.......................................................................................... 35
page 5 of 36
1User Information
1.1 Using this Instruction Manual
Please read this entire instruction for use before operating the simex cuff Mor simex cuff S
device for the first time.
Please read the safety instructions (chapter 1.6) to avoid hazards.
This instruction for use is a component of the simex cuff Mand simex cuff S. Keep this
instruction for use in an easily accessible location.
Include this instruction for use when passing the simex cuff Mor simex cuff S device on to third
parties.
1.2 Icons
1.2.1 General Symbols
Symbol
Meaning
Symbol
Meaning
Attention: possible bodily
injury, health risks or possible
property damage.
NOTE
Note containing useful
information and tips.
Radiofrequency - RF
MRI - Unsafe
1.2.2 Device and Packaging
Symbol
Meaning
Symbol
Meaning
Protect from moisture
Order number
Protection class II
Serial number
Humidity limitation
Lot number
Air pressure limitation
Date of manufacture
Follow the instruction for use
Manufacturer
Protection class: Type BF (Body
Floating)
Do not use if packaging is
damaged!
Temperature limitation
Do not reuse
This device must not be
disposed of in domestic waste.
Power supply unit
Keylock (symbol in display)
Is activated automatically during
operation and can be cancelled by
simultaneously pressing the Up and
Down buttons.
Federal Law restricts this
device to sale or rental by or
on the order of a physician.
page 6 of 36
1.2.3 Display
Symbol
Meaning
Battery full
Battery low
Battery empty
Up
Down
OK (On, Enter)
Cancel (Off, Back)
Power supply unit is connected
Run mode
Pause mode
Filter run time elapsed; replacement of the internal filter by service is required!
Alarm OFF
The alarm “System closed” is inactive.
1.3 Symbol Convention
Symbol
Meaning
•
Enumeration
1.
2.
Perform the process in the specified order.
1.4 Glossary
A
approx.
Abbreviation for “approximately”
Aspirate
Aspirate is the generic term for secretions, bodily fluids and liquids used for
flushing that are typically accumulated when aspirating the upper respiratory
system. It can be easily aspirated using the devices described here.
C
Contamination
Contamination means that bacteria and viruses from the aspirate have come
into contact with the interior of the device.

page 7 of 36
D
DFS®
Double filter system (only simex
cuff S
)
An external filter and a bacterial filter integrated into the aspirator make up the
double filter system. The double filter system effectively protects the interior of
the device from contamination and overflow. It enables safe processing and
rapid reuse of the product.
E
e.g.
For example, abbreviation for Latin “exempli gratia”
I
incl.
IP22
Abbreviation for “inclusive”
International Protection / Protection Class
The Protection Class defines the degree of protection of the device against
contact and ingress of liquids.
The simex
cuff M
and simex
cuff S
are protected against finger access and
falling water drops at an inclination of up to 15°.
O
Overflow
Overflow means that the aspirate is sucked into the interior of the device.
P
Processing
The processing procedure is required for each new patient. The term processing
denotes the process in which parts coming or potentially coming into contact
with aspirate are cleaned, disinfected and replaced if necessary.
The processing procedure must only be performed by simex Medizintechnik
GmbH or an authorized service partner of simex Medizintechnik GmbH.
1.5 Intended Use
The simex cuff Mrespectively simex cuff S device is a network-independent mobile medical
device for subglottic aspiration of secretions to be used with cuffed endotracheal and tracheal tubes
with integrated subglottic suction ports.
The simex cuff Mrespectively simex cuff S must never be used simultaneously on more
than one patient!
1.5.1 Essential Features
Generation of a vacuum
Generation of volumetric flow
Aspiration of secretion
1.5.2 Indications
The simex Subglottic Aspiration System is indicated for vacuum suction, extraction,
aspiration and removal of surgical fluids, tissue (including bone), bodily fluids or
infectious material from wounds or from patient's airway or respiratory system,
either during surgey or at the patient's bedside.
Generally, the simex Subglottic Aspiration System is intended for removing
subglottic secretions from the patient´s airway above the endotracheal or tracheal
cuff using intermittent suction when used in ICU and acute care settings where the
duration of mechanical ventilation is limited to a maximum of 4 weeks.
1.5.3 Contraindications
The simex cuff Mand simex cuff S Subglottic Aspiration System is not intended for
continuous operation in low vacuum drainage (e.g. thoracic, wound drainage)
1.5.4 Restrictions on use
In medical rooms where potential equalization is necessary (e.g. heart surgery)
In hazardous areas
Outside / outdoor

page 8 of 36
1.6 Basic Safety Instructions
CAUTION!
Health risks due to the handling of infectious liquids or pathogenic
germs.
Infectious and pathogenic germs in the aspirate cause health risks.
Always aspirate with endotracheal or tracheal tubes with integrated suction
ports. The suction tube must never come into contact with the aspiration area.
Follow the hygiene, cleaning and decontamination instructions.
WARNING!
Risk of damage due to improper power supply.
Improper operation causes overvoltage in the device which may be transmitted
to the operator.
Ensure prior to startup that the mains supply is designed to operate at supply
voltages of 100-240 V alternating current.
Ensure prior to startup in UL listed markets such as the USA and Canada that
the mains supply is designed to operate at a supply voltage of 120 V
alternating current.
Only operate the device with the provided power supply unit (Type: FRIWO
FW 7555M/12).
ATTENTION!
Risk of damage due to electromagnetic phenomena.
Medical electrical equipment is subject to special precautionary measures
regarding electromagnetic compatibility and must be installed and operated in
accordance with the EMC information provided in the accompanying
documentation! (see chapter 7.3)
CAUTION!
Hazard of persons due to improper handling.
Use the device for its intended purpose only.
Never use the device for wound treatment.
Never use the device for thorax drainage.
When using the power supply unit, make sure the power supply unit is
connected to the mains supply (100 V -240 V AC) only after the power cord
plug of the power supply unit has first been connected to the suction device.
The separation of the power supply unit from the mains supply must occur in
exactly the opposite sequence (first separate the power supply unit from the
mains supply (100 V - 240 V AC) and then the power cord plug from the
suction device).
ATTENTION!
Damage to the device due to improper handling.
Never aspirate flammable, corrosive or explosive liquids or gases.
Do not drop the device.
Do not use the device in case of apparent housing damage.
CAUTION!
Safety defects due to improper accessories and spare parts.
The use of accessories and spare parts other than those recommended by simex
Medizintechnik GmbH may compromise the safety and function of the device.
Damage caused by using non-recommended accessories and spare parts or by
improper use is not covered by warranty in any case.
Only use original accessories and spare parts.
ATTENTION!
Damage to the device by ingress of liquids.
Do not use the device near splashing water.
Do not use the device in damp rooms or while bathing or showering.
Do not allow the power supply unit, plug and display film to get wet.
Never submerge the device in water or other liquids (also not while not in
operation).
ATTENTION!
Damage to the device by heat.
Do not cover the power supply unit.
Keep the device as well as the power cord and power supply unit away from
other heat sources.

page 9 of 36
CAUTION!
Hazard of persons due to strangulation.
People may strangle themselves on the tubing or the power cord.
Store the device incl. accessories in the shipping carton.
To prevent misconnections, always trace the tubing to the point of origin
before connecting to a device or port. Recheck connections and all patient
tubes upon the patient's arrival to a new setting or service .
ATTENTION!
Known or identifiable conditions for medical care within a domestic
environment.
Children and pets must be kept away from the device to ensure that the
device is not knocked over or dropped.
Prior to connecting the power supply unit, ensure that the voltage of the
device corresponds to the domestic power supply.
Do not use the device in damp rooms, baths or showers.
Do not allow the power supply unit, plug and switch unit to get wet.
Never submerge the device in water or other liquids (also do not
submerge while not in operation).
Incident light may effect the readability of the display negatively.
1.7 User Requirements
The simex cuff M or simex cuff S device must only be operated and used by instructed and
trained personnel.
Familiarize yourself with the functions of the simex cuff M or simex cuff S device prior to
startup.
Training on the operation of simex cuff M and simex cuff S is provided by simex Medizintechnik
GmbH or an authorized distribution partner of simex Medizintechnik GmbH. Product training takes
approximately one to two hours and includes an explanation of the design and function of the
device, the handling of the device, the alarm system, the cleaning and disinfection as well as the
procedure to be followed for each new patient and for disposal.
Training should be repeated on a regular basis every 24 months.
Each participant receives a certificate as proof of training.
1.8 Information on Product Liability
The liability for the operation of the device is channeled to the operator in the following cases:
the device is used outside its intended use,
the device is not used in accordance with the instruction for use,
the device is opened by unauthorized personnel,
installation, settings, enhancements, routine maintenance or repairs are performed by
unauthorized personnel,
original accessories and spare parts have not been used
1.9 Material Compatibility
ATTENTION!
Aggressive substances may damage the device and the accessories.
Please follow the cleaning and care instructions (chapter 4.1)

page 10 of 36
2Product Description
2.1 Whole View
2.1.1 simex cuff M
Fig. 1 simex cuff M
A Disposable secretion canister (250 ml) with integrated suction tube
B Canister locking mechanism
C (On) and (Off) buttons
D Display
E and arrow buttons
F
G
simex cuff M device Socket
for power supply unit
2.1.2 simex cuff M product contents
the device simex cuff M
instruction for use
2 x disposable secretion canister (250 ml) with integrated bacterial filter, carbon filter, solidifier
and suction tube
power supply unit (Type: FRIWO FW 7555M/12) incl. country adapter
multilingual charging instructions
instructions for safe handling of battery packs
“Used Medical Device” label and decontamination certificate
test report according to IEC 62353
optional accessories (depending on the order)

page 11 of 36
2.1.3 simex cuff S
Fig. 2 simex cuff S
A Disposable secretion canister system (1,000 ml)
B Holder for external canister
C Connecting tube
D Display
E (Off) buttons and and arrow buttons)
F
G
Control panel ( (On) and
simex cuff S device
Socket for power supply unit
2.1.4 simex cuff Sproduct contents
the device simex cuff S
instruction for use
disposable secretion canister system
(comprising the external canister, disposable liner, holder for external canister, connecting tube
and disposable suction tube (sterile))
power supply unit (Type: FRIWO FW 7555M/12) incl. country adapter
multilingual charging instructions
instructions for safe handling of battery packs
“Used Medical Device” label and decontamination certificate
test report according to IEC 62353
optional accessories (depending on the order)

page 12 of 36
2.2 Product Properties
CAUTION!
Hazard of persons due to improper handling.
Use the device for its intended use only.
Never use the device for aspiration in low vacuum range (e.g. thorax
drainage).
ATTENTION!
Damage to the device due to improper handling.
Never aspirate flammable, corrosive or explosive liquids or gases!
Do not drop the device!
Do not use the device in case of apparent housing damage.
The simex cuff Mrespectively simex cuff Sdevice is a portable aspirator for stationary and
in the medical, subglottic aspiration of secretion in combination with endotracheal and tracheal
tubes with integrated subglottic suction port. It is intended for aspiration in the middle vacuum
range and can be used in the hospitals.
The simex cuff Mand simex cuff Sdevices are lightweight, portable aspirators. They are
operated via the internal battery or via the supplied power supply unit that also can be used to
recharge the battery.
The vacuum is generated by a maintenance free electric motor driven membrane pump. After it is
switched on, the vacuum pump creates a vacuum in the tubing system and disposable secretion
canister, which is used to aspirate fluids (with endotracheal and tracheal tubes with integrated
subglottic suction port). The secretion is directed away from the patient and collected in the
disposable secretion canister. If the disposable secretion canister is full, the device triggers the
“System closed – canister full” alarm via an integrated overflow protection system and stops the
pump. The simex cuff Mand simex cuff S devices must only be operated with the supplied
disposable secretion canister (system).
The provided disposable secretion canister for the simex cuff M as well as the disposable liner and
the suction tube for the simex cuff S are intended for single use.
2.2.1 Disposable secretion canister for simex cuff M
The disposable secretion canister consists of a canister with a connected suction tube. The
disposable secretion canister has an integrated bacterial filter, carbon filter and solidifier. The
hydrophobic bacterial filter integrated in the disposable secretion canister is 99.999% effective
against bacteria and viruses. This integrated filter prevents an overflow in the event of an
operational error. If the liquid reaches this filter, aspiration is no longer possible and the error
message “System closed – canister full” appears on the display. The aspiration process is
discontinued. The disposable secretion canister must be replaced.
The activated carbon filter in the disposable secretion canister reduces the spread of odor.
Solidifier:
Disposable secretion canisters filled with aspirate can be transported and disposed in a leak-proof
manner by using the solidifier. The aspirate solidifies after an average gelling time of 2 to 5
minutes (depending on the consistency of the aspirate), irrespectively of the aspiration intervals.
The disposable secretion canister incl. the suction tube is intended for single use.
Replace the disposable secretion canister in accordance to the respectively applicable
hygiene instructions, if it is full, prior to each new patient or weekly at the latest.
2.2.2 Information on the simex filter system for the simex cuff M
The filter system of the simex cuff M consists of the external bacterial filter integrated in the
disposable secretion canister and the internal filter installed in the device. The internal filter is a
self-sealing bacterial filter and is 99.999% effective against bacteria and viruses in combination
with the integrated filter in the disposable secretion canister.
The simex filter system effectively protects the interior of the device from contamination
and overflow.

page 13 of 36
Service life and reuse
The internal filter is not intended for reuse. To ensure consistent performance, the
internal filter must be replaced after contact with the secretion (blocked), after the filter
service life has expired ( symbol in the display) or during maintenance / repair.
The internal filter must be replaced by simex Medizintechnik GmbH or an authorized service
partner of simex Medizintechnik GmbH.
2.2.3 Information on the carbon filter of the simex cuff M
An additional filter in the exhaust air vent of the simex cuff M removes undesirable odor out of
the exhaust air of the device. This filter consists of a thin activated carbon coated nonwoven. The
activated carbon in the nonwoven adsorbs the odor particles of the exhaust air and neutralizes
them. Spreading of odor will be effectively reduced.
Service life and reuse
The carbon filter is not intended for reuse. To ensure consistent performance, the carbon
filter must be replaced during maintenance / repair or after 2 years at the latest.
The carbon filter must be replaced by simex Medizintechnik GmbH or an authorized service
partner of simex Medizintechnik GmbH.
2.2.4 Disposable secretion canister system for simex cuff S
The disposable secretion canister system consists of the external canister and the disposable liner.
The disposable liner has an integrated bacterial filter, carbon filter and solidifier. The self-sealing
bacterial filter integrated in the disposable liner is 99.999% effective against microorganisms.
This integrated filter prevents an overflow in the event of an operational error. If the liquid
reaches this filter, aspiration is no longer possible and the error message “System closed –
canister full” appears on the display. The aspiration process is discontinued. The disposable
liner must be replaced.
The activated carbon filter in the disposable liner reduces the spread of odor.
Solidifier:
Disposable liners filled with aspirate can be transported and disposed in a leak-proof manner by
using the solidifier. The aspirate solidifies after an average gelling time of 2 to 5 minutes
(depending on the consistency of the aspirate), irrespectively of the aspiration intervals.
The disposable liner and the suction tube are intended for single use. Replace the
disposable liner incl. suction tube in accordance to the respectively applicable hygiene
instructions, if it is full, prior to each new patient or weekly at the latest.
2.2.5 Information on the double filter system for simex cuff S
The simex double filter system DFS® consists of the external bacterial filter integrated in the
disposable liner and the internal filter installed in the device. The filters are hydrophobic and self-
sealing bacterial filters, which, in combination, are 99.999% effective against bacteria and viruses.
The internal bacterial filter is installed in the simex
cuff S
.
The external bacterial filter is incorporated in the disposable liner.
The simex double filter system DFS® effectively protects against overflow and contamination
of the interior of the device. It permits fast, simple and cost-effective maintenance.
Service life and reuse
The internal filter of the simex double filter system DFS® as well as the disposable liner are
not intended for reuse. To ensure consistent performance, the internal filter must be
replaced after contact with the secretion/aspirate (blocked), after the filter service life has
expired ( symbol in the display) or during maintenance / repair.
The internal filter must be replaced by simex Medizintechnik GmbH or an authorized service
partner of simex Medizintechnik GmbH.
page 14 of 36
2.2.6 Battery
The charge level of the battery is shown in the display.
It is strongly recommended to fully charge the battery prior to first startup of the simex cuff M
respectively simex cuff S and to repeat this after the first uses.
The simex cuff M and simex cuff S are equipped with a lithium-ion battery, which, unlike
traditional types of rechargeable batteries, has a low self-discharge rate.
The simex cuff M and simex cuff S device should ideally be stored and charged at room
temperature in accordance with the ambient conditions specified in the technical data. Never store
the device incl. battery in a discharged state!
Fully recharge the battery if the device is not operated for a longer period of time (approx. 10
months).
Lithium-ion rechargeable batteries do not have a memory effect. They can, therefore, be recharged
at any time after initial charging.
Only frequent short-time charging should be avoided.
The battery of the simex cuff M and simex cuff S is protected against deep discharge, but the
charging information listed above must nevertheless be followed. The battery is also protected
against overheating during charging. If the battery temperature is exceeded during charging due to
improper ambient conditions, charging is temporarily discontinued to allow cooling. The purpose of
this measure is to ensure safe operation and to protect the battery.
The operational service life of the battery is 2 years. According to the manufacturer of the battery,
the battery has a remaining capacity of more than 80% after 300 charge cycles.
2.2.7 Pressure settings
Once the simex cuff Mor simex cuff S has been switched on, the pressure settings can be
individually adjusted by a healthcare professional.
The pressure settings can be adjusted in a range from -60 mbar to -300 mbar (in steps of
10 mbar). -120 mbar is factory preset pressure.
The change of pressure setting can also be performed during operation.
Always use the lowest possible pressure setting. Adjustments to device settings
must only be made if instructed to do so and only by healthcare professionals.
Prior to switching on the simex cuff Mor simex cuff S it must be ensured that
the device is equipped with a disposable secretion canister.
Follow the maximum pressure setting recommended by the manufacturer of
endotracheal (ETT) or tracheal (TT) tube with integrated suction lumen. In
addition follow the manufacturers instructions for use of the ETT and TT tubes
used with simex cuff M and cuff S.
2.3 Warranty
The devices of simex Medizintechnik GmbH are covered by warranty for 2 years. It is neither
extended nor renewed by warranty work.
The battery is covered by warranty for 6 months.
Wearing parts are excluded from the warranty.
simex Medizintechnik GmbH is responsible for impacts on safety, reliability and specified
performance only if:
original simex accessories and spare parts are used,
maintenance and repair are performed by professionals authorized by simex Medizintechnik
GmbH or by simex Medizintechnik GmbH itself,
the affected product is used and operated in accordance with the instruction for use and within
its intended use.
CAUTION!
page 15 of 36
simex Medizintechnik GmbH does not warrant accurate function of the devices simex cuff M and
simex cuff S and is not liable for a loss of property or personal injury in the following
circumstances:
no original simex accessories or spare parts are used,
using information of this instruction for use are ignored,
installation, settings, changes, upgrades or repairs are not carried out by simex Medizintechnik
GmbH or by professionals authorized by simex Medizintechnik GmbH,
the safety seal is broken or removed.
All warranty claims are voided if the device is opened by unauthorized personnel, the
safety seal is removed / damaged or repairs have been performed by unauthorized
personnel.
3Operation
CAUTION!
Hazard of persons due to improper handling.
Use the device for its intended purpose only.
Read chapter 3.1 to 3.4!
Perform the aspiration in the respiratory area only after instruction by trained
personnel!
Use exclusively endotracheal and tracheal tubes with suction port for aspiration!
ATTENTION!
Malfunction due to aspirated secretions.
Ensure that the disposable secretion canister (250 ml) of the simex cuff M
and the disposable liner (1,000 ml) of the simex cuff S is replaced on a
regular basis. If the disposable secretion canister respectively the disposable
liner is full, the integrated overflow protection system is triggered and the
alarm “System closed – canister full” is activated. This disrupts the aspiration
process.
Switch off the device when replacing the disposable secretion canister
respectively the disposable liner.
If the internal filter of the simex cuff M or the DFS® of the simex cuff S is
blocked, the device must be properly processed by simex Medizintechnik
GmbH or by an authorized service partner of simex Medizintechnik GmbH!
WARNING!
Hazard of persons during operation in a domestic environment.
Due to the increasing mobility of patients in a domestic environment, there is an
increasing risk of forming leaks or blockages in the tube system.
For this reason, a detailed training and instruction of patients as well as
performing regular monitoring of the tubing and aspiration system by trained
personnel is mandatory.
3.1 Set-Up and Startup
3.1.1 Startup
It is important to follow the safety instructions in chapter 1.6 prior to initial startup.
Always have one backup disposable secretion canister (250 ml) for the simex cuff M and one
backup disposable liner (1,000 ml) for the simex cuff Sready, since it is absolutely necessary
for safe operation!
Remove the device and the accessories from the packaging.
Please read this entire instruction for use before operating the simex cuff Mor simex cuff S
device for the first time.
Always place the device on a sturdy and flat surface, take care of the correct position of the
device.
Fully charge the battery prior to initial startup.
Inspect all tubings as well as the power supply unit for damage prior to each startup of
the simex cuff Mrespectively simex cuff S. It is important to avoid kinking when connecting
the tubing. Ensure prior to switching on the unit that the disposable secretion canister and
tubings are properly connected.
Perform a function test! (Please refer to chapter 5.1)
page 16 of 36
3.1.2 Connecting the simex cuff M and simex cuff S
Use the socket for power supply unit of the simex cuff M (chapter 2.1.1, fig. 1 (G)) or the socket
for power supply unit of the simex cuff S (chapter 2.1.3, fig. 2 (G)) to connect the device to the
mains power supply via the supplied power supply unit (type: FRIWO FW 7555M/12) for charging
or operation as required.
Use the supplied power supply unit only. First connect the power supply unit to the socket
for power supply unit of the simex cuff M or simex cuff S and then to the mains power supply.
Fig. 3 Connecting the simex cuff M and simex cuff S to the patient and accessories
3.1.3 Positioning of the simex cuff M
The simex cuff M can be placed next to the patient's bed or attached by means of a variable
holder for tube and rail systems. An optional carrying bag is available for portable use. It is,
however, up to the physician to decide whether the condition of the patient permits portable
use. The simex cuff M can also be used in a horizontal position:
Fig. 4 simex cuff M horizontally
To ensure optimum aspiration of the secretions, place the simex cuff M below the
suction point. It should be noted that the suction tube does not form a dip/loop and is
situated at least on patient level.

page 17 of 36
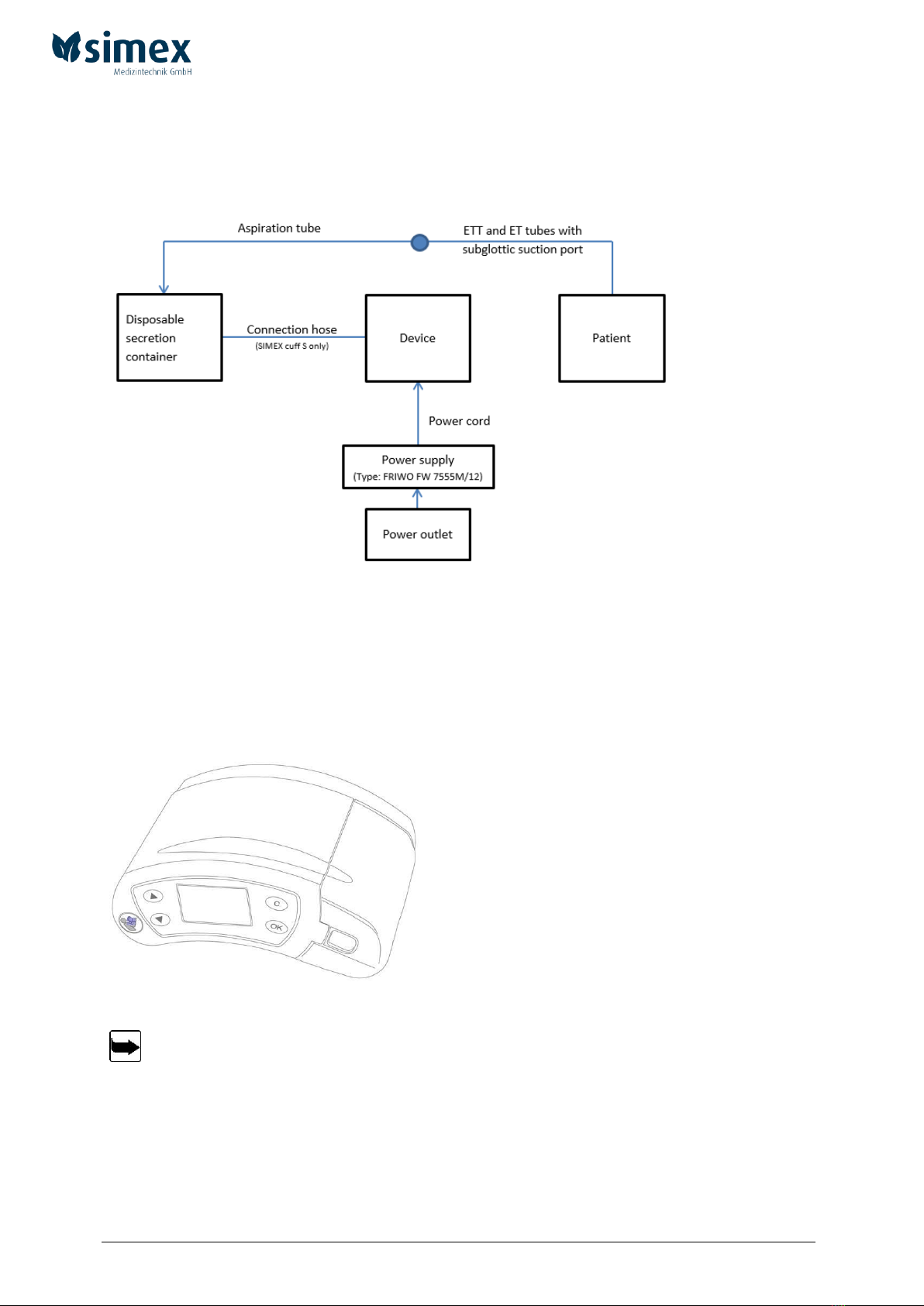
3.1.4 Connecting the disposable secretion canister (250 ml) of the simex cuff M
Fig. 5 Connecting the disposable secretion canister
A
B
C
D
E
Disposable secretion canister (250 ml) incl. suction tube
Locking mechanism for canister
Aspiration port
simex
cuff M
Guiding rail
1. Remove the disposable secretion canister (250 ml) (fig. 5 (A)) from the packaging.
2. Slide the canister on the guiding rails (fig. 5 (E)) of the simex cuff M until the disposable
secretion canister clicks into place in the locking mechanism (fig. 5 (B)).
3. Connect integral suction tube of canister with a sterile suction tube to ETT or ET suction port.
3.1.5 Positioning of the simex cuff S
The simex cuff S can be placed next to the patient's bed. Optionally, a variable holder
for attachment of the device to tube and rail systems as well as a bed holder is available.
3.1.6
To ensure optimum aspiration of the secretions, place the simex cuff S below the
suction point. It should be noted that the suction tube does not form a dip/loop and is
situated at least on patient level.
Connecting the simex disposable secretion canister system of the simex cuff S
ATTENTION!
Malfunction due to collapsing disposable liner.
A leak in the external canister or at the lid of the disposable liner may cause air
to flow into the external canister. This may lead to the collapse of the disposable
liner.
Inspect the disposable secretion canister system (1,000 ml) to ensure that
the lid of the disposable liner is firmly connected to the external canister.
Ensure that all connections are firmly attached and properly connected.
Ensure that the external canister is undamaged and the T-piece is firmly
attached.
Follow the instruction for use supplied by the manufacturer!
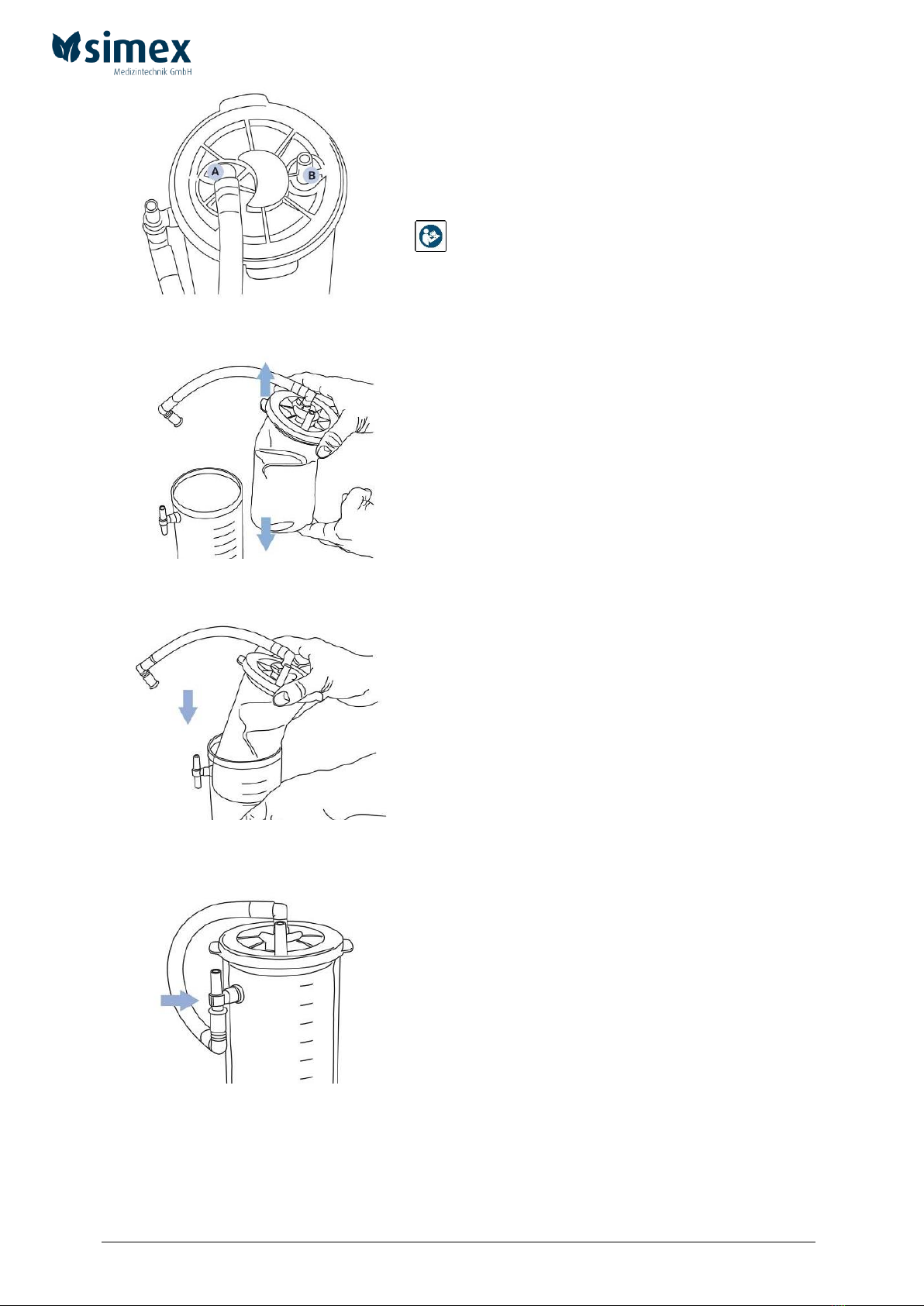
The original simex disposable secretion canister system consists of the external canister, the holder
for the external canister, the disposable liner, the connecting tube for the disposable liner and the
sterile disposable suction tube with step connector.
page 18 of 36
Connection designation
A Vacuum connection
B Patient connection
Please also follow the instruction for use
supplied with the disposable secretion
canister system (1,000 ml)!
Fig. 6
1. Remove the disposable liner from the packaging and fully extend it.
Fig. 7
2. Place the disposable liner in the reusable external canister. Press the lid’s edges firmly
down to ensure proper sealing.
Fig. 8
3. Attach the prefitted connecting tube of the disposable liner to the bottom end of the T-
piece located at the external canister.
Fig. 9
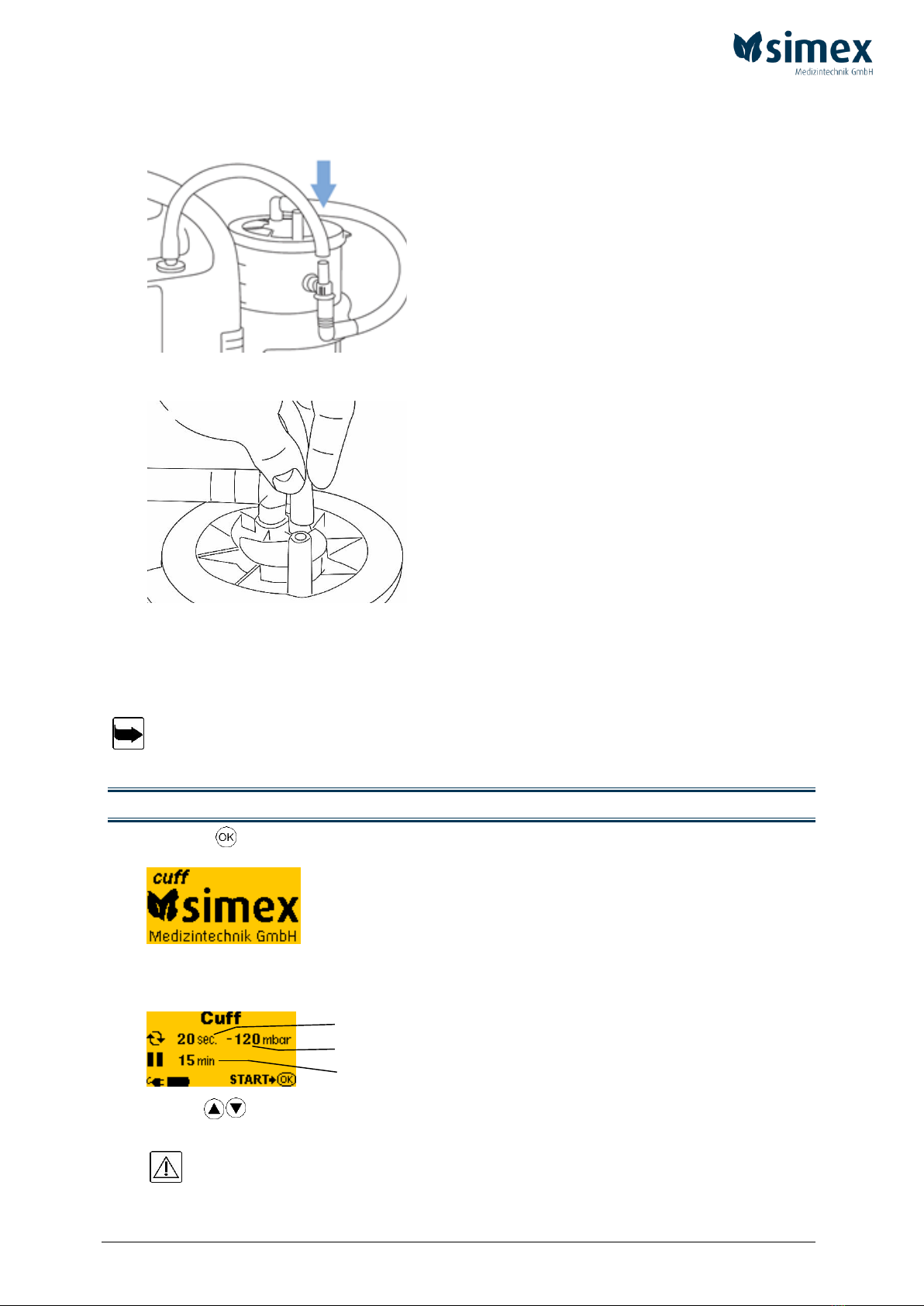
page 19 of 36
4. Connect the vacuum connection of the device with the corresponding vacuum connection
of the external canister (top end of the T-piece). Use the supplied connecting tube to do
so.
Fig. 10
5. Connect the patient connection of the disposable liner (fig. 6 (B)) to the suction tube.
Fig. 11
3.1.7 Connecting endotracheal and tracheal tubes with integrated suction port
Connect the suction tube of the disposable secretion canister to the integrated suction port of
endotracheal and tracheal tubes.
The suction tube must never come into direct contact with the aspiration area.
3.2 Operation of the simex cuff M and simex cuff S
1. Press the button for 1-2 seconds to switch on the simex cuff Mor simex cuff S. The
following start screen is displayed for 5 seconds:
2. The following screen is displayed:
(preset of the target value: -120 mbar)
Run time
Target value
Pause time
3. Use the arrow buttons to set the prescribed vacuum value (target value).
Maximum pressure must not exceed -200mbar or -150mmHg. The
recommended guidelines for pressure ranges for adults is -106 to -200mbar or
-80 to -150 mmHg. The pressure setting in children is not known, but should
not exceed -106mbar or -80 mmHg. In case of blockage of secretion fluids in
the suction lumen of ETT or TT cuffed tubes, the medical professional may
increase the pressure settings from between -200 up to -300mbar to clear the
blockage and then return back to the recommended lower pressure settings.
CAUTION!

page 20 of 36
4. Press the button to start the therapy. 2 values are shown in the display.
5.
The bar in the upper display section moves from the right to the left and shows the pause time.
Press the button to stop the therapy.
6. You will get back to the overview screen:
7. Switch off the simex cuff Mor simex cuff S by pressing the button for 3 seconds.
To aspirate without pause in case of a high rate of secretion or during flushing, press the
button two times at the beginning of the pause time to skip the pause. If necessary repeat
this step.
3.2.1 Setting the run and pause time
The simex cuff M and simex cuff S enable the selection of run and pause time at initial startup. The
selected values are stored and automatically loaded at each startup. To customize the time
settings, follow these steps:
1. Press the button for 1-2 seconds to switch on the simex cuff Mor simex cuff S. The
following start screen is displayed for 5 seconds:
2. While the start screen is displayed, simultaneously press the arrow buttons. The menu
Setup is displayed.
3. Select the menu Parameters with the arrow buttons.
4. Use the button to confirm your choice.
Actual value
Target value
The bar in the upper display section fills in from the left to the right and shows the run time.
The pause time follows subsequent to the run time.
This manual suits for next models
1
Table of contents