Smiths Medical CADD 2120 User manual

CADD®-Solis VIP
Ambulatory Infusion Pump
Technical Manual
Model 2120
®
s
X

2
e issue date of this Technical Manual is included on the back cover. In the event one year has elapsed
between the issue date and product use, contact Smiths Medical to see if an updated revision of this
manual is available.
Technical Assistance
For detailed instructions, specications, warnings, warranties, and additional information on operating
the CADD®-Solis VIP ambulatory infusion pump, refer to the Operator’s Manual supplied with the
product.
Smiths Medical is available to help with the programming and operation of the CADD®-Solis VIP
ambulatory infusion pump. If you have comments or questions, call the number given below. When
calling, specify the pump’s soware version number. is information is located in the Device Information
Report (see the Reports section in the CADD®-Solis VIP Operator’s Manual for more information).
Your facility may load a unique single protocol or protocol library on the CADD®-Solis VIP ambulatory
infusion pump. When a pump is returned to Smiths Medical for service, the protocol or protocol library
may be cleared as part of the service or repair process. If this occurs, the pump will be labeled with
a sticker indicating the change. Before returning the pump to use, follow your facility’s policies and
procedures to load the correct protocol or protocol library back onto the pump. If necessary, contact your
CADD®-Solis administrator or Smiths Medical for assistance.
U.S. Distribution:
Smiths Medical ASD, Inc.
St. Paul, MN 55112 USA
1 800.258.5361 (USA)
1 214.618.0218
www.smiths-medical.com
European Distribution:
Smiths Medical International Ltd.
TN25 4BF, UK
+44 (0) 1233 722100
Australian Distribution:
Smiths Medical Australasia Pty. Ltd.
61 Brandl Street
Eight Mile Plains, QLD 4113, Australia
+61 (0) 7 3340 1300

3
Table of Contents
Technical Assistance ...........................2
Introduction ............................ 4
Limited Warranty .................................4
Warnings .........................................4
Cautions..........................................5
Pump Overview ......................... 6
Delivery Modes...................................6
PCA Delivery Prole ..........................6
Continuous Delivery Prole ..................6
Intermittent Delivery Prole..................7
Step Delivery Prole .........................7
Taper Delivery Prole ........................7
Pump Diagram ...................................8
PCA Delivery Mode Scroll Ranges.................9
Specications (Nominal) .........................11
General Pump Specications................11
Delivery Specications ......................14
Remote Dose Cord ......................... 16
Batteries............................... 17
Recommended Batteries ........................17
Replacing the Battery Door ......................17
CADD®-Solis Rechargeable Battery Pack .........17
Battery Storage..................................17
Battery Life ......................................17
Collect Separately .............................. 20
Construction...........................21
Pumping Operation ....................22
Battery Backed RAM ............................ 22
LCD Circuitry ................................... 22
LED Status Indicators ........................... 22
Audible Alarm Circuitry ......................... 22
Power Circuitry ................................. 22
Battery Status .................................. 23
Pumping Mechanism ........................... 23
Pumping Characteristics........................ 24
Cassette “Type” Sensor Circuit.............. 24
Latch/Lock Sensor Circuit .................. 24
Hardware and Software Fault Detection 25
System Fault Alarm............................25
Order of System Fault Alarm Events .......25
Cleaning Procedures.................. 26
Cleaning Solutions ............................26
Cleaning the Pump and Accessories ...........26
Annual Inspection and
Testing Procedures ................... 27
Inspection Recommendation..................27
I. Visual Inspection ...........................27
II. Mechanical Inspection .....................27
III. Functional Testing .........................28
IV. Occlusion Pressure Range Tests ............33
V. Accuracy Tests .............................35
Pump Cleaning and Functional Testing
Checklist............................. 39

4
IntroductIon
Introduction
is Technical Manual is applicable only to the model 2120 CADD®-Solis VIP ambulatory infusion pump.
It is intended to provide a basic but limited understanding of the mechanical and electrical operation
of the CADD®-Solis VIP ambulatory infusion pump to people familiar with the device. It also outlines
cleaning and functional testing procedures that can be performed on the pump. e CADD®-Solis VIP
Operator’s Manual should be used in conjunction with this manual for complete information.
IMPORTANT NOTICE:
CADD®-Solis VIP ambulatory infusion pump operations and safety features are based on a
microcomputer design. Inadequate servicing or tampering with the safety features of the pump may
seriously aect performance and safety. For that reason, ALL SERVICING AND REPAIR OF THE
CADD®-SOLIS VIP AMBULATORY INFUSION PUMP MUST BE PERFORMED BY SMITHS
MEDICAL OR ITS AUTHORIZED AGENTS.
e manufacturer’s warranty agreement shall become null and void if the pump is not used in accordance
with the Operator’s Manual and Instructions for Use provided with the pump accessories; or if the pump
is serviced by anyone other than Smiths Medical or those authorized by Smiths Medical.
Limited Warranty
e limited warranty associated with the CADD®-Solis VIP ambulatory infusion pump can be found in
the product literature supplied with the product when originally purchased, which is incorporated herein
by reference. SMITHS MEDICAL SPECIFICALLY DISCLAIMS ANY OTHER WARRANTY, WHETHER
EXPRESS, IMPLIED OR STATUTORY, INCLUDING, WITHOUT LIMITATION ANY IMPLIED
WARRANTY OF MERCHANTABILITY OR FITNESS FOR USE. Smiths Medical further disclaims
any responsibility for the suitability of the system for a particular medical treatment or for any medical
complications resulting from the use of the system.
e manufacturer shall not be responsible for any incidental damages or consequential damages to
property, loss of prots, or loss of use caused by any defect or malfunction of the system.
If you wish to receive additional information about the extent of the warranty on these products, contact
your Smiths Medical representative or call Customer Service at 1800.258.5361 (USA) or +1214.618.0218.
All recommendations, information, and literature supplied by Smiths Medical with respect to the
CADD®product line are believed to be accurate and reliable, but do not constitute warranties. No agent,
representative, or employee of Smiths Medical has authority to bind Smiths Medical to any representation
or warranty, expressed or implied.
Warnings
•e user should ensure that the performance oered by the pump is t for the intended use and that the
pump is not used in any way or for any purpose other than its intended use.
•If the pump is dropped or hit, inspect it for damage. Do not use a pump that is damaged or not
functioning properly. Contact Smiths Medical Customer Service to return a pump for service.
•Do not use rechargeable NiCd or nickel metal hydride (NiMH) batteries. Do not use carbon zinc
(“heavy duty”) batteries. ey do not provide sucient power for the pump to operate properly.
•Always check the battery compartment for uid or debris before inserting the batteries, and do not allow
any uid or debris to fall into the battery compartment. Fluid or debris in the battery compartment may
damage the battery contacts, and could result in loss of power and nondelivery of drug.
•Residential/facility wiring must comply with all applicable electrical codes. Do not bypass power cord
connections. Do not remove a prong from the power cord.
•Ensure that the ± 6% system delivery accuracy specication is taken into account when programming
the pump and/or lling the reservoir. Failure to do so may result in medication in the reservoir
becoming depleted sooner than expected.

5
IntroductIon
•System delivery inaccuracies beyond ± 6% may occur as a result of back pressure or uid resistance,
which depends upon temperature, drug viscosity, catheter size, extension set tubing (for example,
microbore), in-line components (such as lters and needleless access connectors), and placing the
infusion reservoir and/or pump above or below the level of the patient. System delivery inaccuracy may
result in under or overdelivery of medication.
•ere are potential health hazards associated with improper disposal of batteries, electronics, and
contaminated (used) reservoirs and extension sets. Dispose of used batteries, reservoirs, extension sets,
and other used accessories, or a pump that has reached the end of its useful life, in an environmentally
safe manner, and according to any regulations that may apply.
•Ensure that debris is not allowed to build up on the pressure plate surface of the pumping mechanism.
Inspect the air detector sensor slot and remove any debris. A blocked air detector sensor may not detect
air present in the uid path.
Cautions
•Do not operate the pump at temperatures below 2°C (36°F) or above 40°C (104°F) to avoid damaging
the electronic circuitry.
•Do not store the pump at temperatures below –20°C (–4°F) or above 60°C (140°F) to avoid damaging
the electronic circuitry. Do not store the pump with a CADD™medication cassette reservoir or CADD®
administration set attached.
•Do not expose the pump to humidity levels below 20% or above 90% relative humidity to avoid
damaging the electronic circuitry.
•Do not store the pump for prolonged periods with the batteries installed. Battery leakage could damage
the pump.
•Do not twist or turn the remote dose cord connector, or use any instrument to remove it from the pump.
•Inspect the AA batteries for damage or wear to the metal or plastic insulation prior to use, or aer the
pump has been dropped or hit. Replace the batteries if any damage is noted.
•If the power up results in an error message indicating that the protocol library was lost, do not proceed
with using the pump. Follow your facility’s procedures for downloading protocol libraries.
•Do not clean the pump with acetone, other plastic solvents, or abrasive cleaners, as damage to the
pump may occur.
•Do not immerse the pump in cleaning uid or water. Do not allow solution to soak into the pump,
accumulate on the keypad, or enter the battery compartment, USB port, remote dose cord jack, or
power jack areas. Moisture buildup inside the pump may damage the pump.
•CADD®pumps are sealed units. A broken or damaged seal will therefore be considered conclusive
evidence that the pump has been misused and/or altered, which voids any and all warranties. All
service and repair of CADD®pumps must be performed by Smiths Medical or its authorized agents.
•At the completion of the Downstream Occlusion Pressure Range Test 2, the pressure must be reduced
to zero before detaching the cassette from the pump; otherwise, the cassette may rupture. Safety glasses
should be worn while conducting or observing this test.
6
PumP overvIew
Pump Overview
Delivery Modes
e CADD®-Solis VIP ambulatory infusion pump system provides measured drug therapy to patients
in hospital or outpatient settings. e CADD®-Solis VIP ambulatory infusion pump is indicated
for intravenous, intra-arterial, subcutaneous, intraperitoneal, in close proximity to nerves, into an
intraoperative site (so tissue, body cavity/surgical wound site), epidural space, or subarachnoid space
infusion.
is pump is not to be used in any intra-articular space infusion.
Epidural administration is limited to short-term infusion of anesthetics, and either long- or short-term
infusion of analgesics.
Subarachnoid administration is limited to short-term infusion of analgesics.
PCA Delivery Prole
PCA (patient controlled analgesia) delivery is used for therapies that require a continuous rate of infusion,
patient-controlled demand doses, or both, such as patient-controlled analgesia.
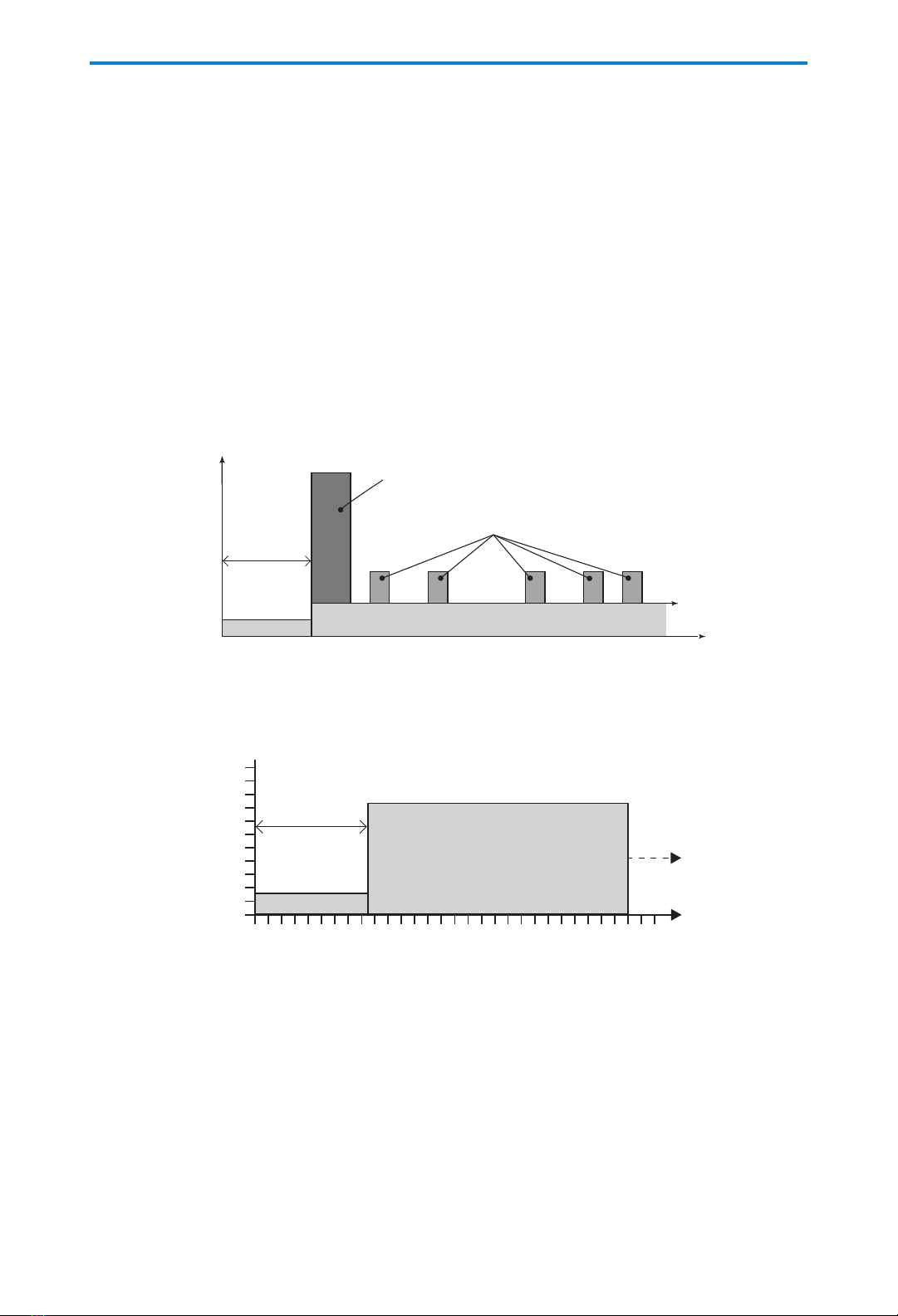
Continuous Delivery Prole
Continuous delivery allows the infusion of drug/uid at a constant, programmed rate.
Clinician Bolus
(used here as a loading dose)
PCA Doses
Continuous Rate
Time
Dosage
Delayed start
(optional)
KVO
Time
Delivery Rate
Delayed start
(optional)
KVO
Continuous Rate

7
PumP overvIew
Intermittent Delivery Prole
Intermittent delivery allows the infusion of a specic volume of drug/uid at a regular, programmed
interval.
Step Delivery Prole
Step delivery allows an incremental increase in infusion rate to a specied maximum infusion rate for a
specied total infusion volume.
Taper Delivery Prole
Taper delivery allows a plateau rate of infusion with the option of tapering at the beginning and/or end
and has a programmable KVO rate at the end of the infusion.
Time
Delivery Rate
Delayed start
(optional)
KVO
KVO Dose
Dose
Duration Duration
Cycle
KVO
Time
Delivery Rate
Plateau rate
Initial rate
Rate (step)
increase
Delayed
start
(optional)
KVO
Rate (step)
increase
KVO
Infusion volume
Step
duration
Infusion Duration
Plateau Rate
Taper
Up
Taper
Down
Infusion Volume
Time
Delivery Rate
Delayed start
(optional)
KVO KVO

8
PumP overvIew
Pump Diagram
Front View
Rear View
Battery Compartment
Display
Indicator Lights
Amber
Green
AC Power Jack
Keypad
Blue AC
Power Light
Remote Dose
Cord Jack
USB Port
Power Switch
Cassette/Keypad Lock
(Unlock/Lock)
Cassette Latch
Cassette
(The part of the CADD™ medication cassette
reservoir or CADD® administration set that
attaches to the pump)
Serial
number
9
PumP overvIew
PCA Delivery Mode Scroll Ranges
PCA Continuous Rate Scroll Ranges
Units Starting Value Increment Maximum
Milliliters 00.10 100.00
Milligrams
only
10% of
concentration
Values between 0.01 and 0.5: 0.01 Concentration x 100
Micrograms
only
10% of
concentration
Values between 0.1 and 0.5: 0.1 Concentration x 100
Milligrams
and
Micrograms
10% of
concentration
Values between 0.5 and 100: 0.1 Concentration x 100
Values between 100 and 1000: 1.0
Values greater than 1000: 10.0
PCA Dose and Clinician Bolus Scroll Ranges
Units Starting Value Increment Max.
Milliliters 00.05 50
PCA Dose and Clinician Bolus Scroll Ranges: Milligrams
Concentration
(mg/mL)
Increment
(mg)
Max.
(mg)
Concentration
(mg/mL)
Increment
(mg)
Max.
(mg)
0.1 0.01 520 1.00 1000
0.2 0.02 10 25 1.25 1250
0.3 0.03 15 30 1.50 1500
0.4 0.04 20 35 1.75 1750
0.5 0.05 25 40 2.00 2000
10.05 50 45 2.25 2250
20.10 100 50 2.50 2500
30.15 150 55 2.75 2750
40.20 200 60 3.00 3000
50.25 250 65 3.25 3250
60.30 300 70 3.50 3500
70.35 350 75 3.75 3750
80.40 400 80 4.00 4000
90.45 450 85 4.25 4250
10 0.50 500 90 4.50 4500
11 0.55 550 95 4.75 4750
12 0.60 600 100 5.00 5000
13 0.65 650
14 0.70 700
15 0.75 750
10
PumP overvIew
PCA Dose and Clinician Bolus Scroll Ranges: Micrograms
Concentration
(mcg/mL)
Increment
(mcg)
Max.
(mcg)
Concentration
(mcg/mL)
Increment
(mcg)
Max.
(mcg)
10.05 50 35 1.75 1750
20.10 100 40 2.00 2000
30.15 150 45 2.25 2250
40.20 200 50 2.50 2500
50.25 250 55 2.75 2750
60.30 300 60 3.00 3000
70.35 350 65 3.25 3250
80.40 400 70 3.50 3500
90.45 450 75 3.75 3750
10 0.50 500 80 4.00 4000
11 0.55 550 85 4.25 4250
12 0.60 600 90 4.50 4500
13 0.65 650 95 4.75 4750
14 0.70 700 100 5.00 5000
15 0.75 750 200 10.00 10,000
20 1.00 1000 300 15.00 15,000
25 1.25 1250 400 20.00 20,000
30 1.50 1500 500 25.00 25,000

11
PumP overvIew
Specications (Nominal)
General Pump Specications
Used to test the
pump
•CADD™medication cassette reservoirs, <21-7002, 21-7308, 21-7302
•CADD®extension sets, <21-7045, 21-7046, 21-7047
•CADD®administration sets, <21-7091, 21-7034, 21-7021, 21-7321
•CADD®high volume administration sets, <21-7057, 21-7357
Resolution •CADD™ medication cassette reservoir: 0.050 mL per pump stroke nominal
•CADD®administration set: 0.050 mL per pump stroke nominal
•CADD® high volume administration set: 0.1 mL per pump stroke nominal
Size Excluding cassette and accessories:
4.1 cm × 10.2 cm × 12.7 cm
1.6 in × 4 in × 5in
Weight Including 4 AA alkaline batteries, excluding other accessories:
595 g
21 oz
Pump alarms •High priority alarms: Air in line detected, Battery depleted while delivering,
Battery removed while delivering, Battery unusable while delivering,
Disposable attached improperly, Disposable detached while delivering,
Disposable locked but not latched, Disposable type high ow administration
set not allowed, Disposable type high ow administration set required,
Disposable type invalid, Downstream occlusion, Key stuck, Pressure sensor
faulty, Pump automatically stopped, Rechargeable battery end of life,
Remote dose cord key stuck, Reservoir volume empty, Stop mode reminder,
Upstream occlusion
•Medium priority alarms: 23
•Low priority alarms: 10
•Informational messages/alerts: 23
Battery fallout
alarm
Alarm sounds for 2 minutes if the pump has been powered up for a minimum
of 4minutes. Note: Alarm enabled while pump is in run mode only.
Classication CF J
Class II K
Moisture
protection Splashproof ( E) per IEC 60529
Maximum infusion
pressure
1.86 bar
27.0 psi

12
PumP overvIew
Maximum time to
occlusion alarm
and Bolus volume
at occlusion alarm
Flow
Rate
(mL/hr)
Tubing Set
Max. Time to
Occlusion
Bolus at
Occlusion
Raw
Test
Data
(min)
Spec.
(min)
Raw
Test
Data
(mL)
Spec.
(mL)
0.1
CADD™ medication cassette
reservoir <21-7002 with
CADD®extension set
<21-7045
90 ≤ 160 0.107 ≤ 0.25
CADD®administration set
<21-7091 122 ≤ 190 0.139 ≤ 0.30
CADD®high volume
administration set <21-7055 1140 ≤ 1200 1.250 ≤ 1.40
Flow
Rate
(mL/hr)
Tubing Set
Max. Time to
Occlusion
Bolus at
Occlusion
Raw
Test
Data
(sec)
Spec.
(sec)
Raw
Test
Data
(mL)
Spec.
(mL)
150
CADD™ medication cassette
reservoir <21-7002 with
CADD®extension set
<21-7045
4≤ 45 0.069 ≤ 0.25
CADD®administration set
<21-7091 4≤ 45 0.072 ≤ 0.30
CADD®high volume
administration set <21-7055 32 ≤ 90 1.059 ≤ 1.40
Power sources •AC adapter
•CADD®-Solis rechargeable battery pack
•Four AA alkaline batteries (for example, Duracell®PC1500/MN1500, IEC LR6)
Charging system
for internal
memory backup
battery
The internal memory backup battery uses lithium manganese dioxide
technology. It charges whenever the pump is powered on and has a 10-month
memory capacity once it has been charged for 250 hours at 20°C (68°F).
System operating
temperature
15°C to 40°C
59°F to 104°F
System storage
and transportation
temperature
–20°C to 60°C
–4°F to 140°F
Relative humidity 20% to 90% relative humidity, non-condensing
Atmospheric
pressure
70 kPa to 106 kPa
10.2 psi to 15.4 psi
13
PumP overvIew
System delivery
accuracy
± 6% (nominal). At low infusion rates, this accuracy may not be achieved for
short periods. During the total infusion time, the accuracy averages out.
WARNING:
•Ensure that the ± 6% system delivery accuracy specication is taken into
account when programming the pump and/or lling the reservoir. Failure to
do so may result in medication in the reservoir becoming depleted sooner
than expected. If the pump is being used to deliver critical or life sustaining
medication, the interruption in the delivery of medication could result in
patient injury or death.
•System delivery inaccuracies beyond ± 6% may occur as a result of back
pressure or uid resistance, which depends upon temperature, drug
viscosity, catheter size, extension set tubing (for example, microbore),
in-line components (such as lters and needleless access connectors), and
placing the infusion reservoir and/or pump above or below the level of the
patient. System delivery inaccuracy may result in under or overdelivery of
medication.
Using CADD™
medication
cassette reservoirs
± 6% (nominal) at 15°C to 40°C with no back pressure
•An additional ± 2.5% change may be seen at ± 100 mmHg (± 1.9 psi).
Using CADD®
administration sets
± 6% (nominal) at 15°C to 40°C with no back pressure.
•An additional ± 2.5% change may be seen at ± 100 mmHg (± 1.9 psi).
Using CADD®
high volume
administration sets
± 6% (nominal) at 15°C to 40°C with no back pressure
•An additional ± 5% change may be seen at ± 100 mmHg (± 1.9 psi).
System denition CADD®-Solis pump with 1 of the following attached:
•Medication cassette reservoir and CADD®extension set
•Medication cassette reservoir with Flow Stop feature and CADD®extension set
•CADD® administration set
•CADD®administration set with Flow Stop feature
High pressure
alarm threshold
1.24 bar ± 0.62 bar
18 ± 9 psi
Air detector alarm Sensitivity:
•Low: Single bubble >400 μL
•High: Single bubble >150 μL
Accumulated Air: Greater than 1 mL air over 15minutes (nominal)
Bolus accuracy
specication: ± 6%
Actual test data for bolus accuracy at 0.05 mL:
Average 0.0508 mL
% Error 1.6%
Minimum Error % –3.0%
Maximum Error % 4.2%
Actual test data for bolus accuracy at 50 mL:
Average 50.77 mL
% Error 1.55%
Minimum Error % –0.07%
Maximum Error % 2.35%
Maximum volume
infused under
single-fault
conditions
•CADD®administration set: 0.15 mL
•CADD®high volume administration set: 0.30 mL

14
PumP overvIew
Delivery rate
during priming
•Standard volume administration set: approx. 250 mL/hr
•High volume administration set: approx. 500 mL/hr
Alarm disabled
during priming
Air-In Line
Delivery Specications
Common Delivery Specications
Reservoir volume 0 to 9999
Programmable in 1 mL increments.
Displayed in 0.1 mL increments.
Given 0 to 99,999.99 in 0.01 unit increments
Res vol low trip
point
1 to 999 mL in increments of 1 mL
Res vol empty
alarm
•Insistent and one time only
•Non-insistent and repeating
Delayed start 0 to 96 hr in 5 min increments
Pump stopped
alarm
•Informational
•High priority
Air detector •On
•O
Air detector
sensitivity
Low Sensitivity: Single bubble > 400μL
High Sensitivity: Single bubble > 150μL
Alarm volume •High
•Medium
•Low
PM (preventive
maintenance)
reminder
Interval: 1 to 24 months in 1 month increments
Enable: On or o
Custom keypad
code
001 to 899 in increments of 1
Custom clinician
code
001 to 899 in increments of 1
Custom
administrator code
001 to 899 in increments of 1
Date format •US standard (month/day/year)
•European standard (day/month/year)
•International standard ISO 8601:2004 (year/month/day)
Time format •00:00 to 23:59 military
•12-hour am/pm
Downstream
occlusion
sensitivity
High Sensitivity: When the high pressure alarm threshold is reached, the
downstream occlusion alarm is triggered immediately.
Low Sensitivity: When the high pressure alarm threshold is reached, the
downstream occlusion alarm is delayed for 2 seconds. This allows for the
pressure to stabilize before a possible alarm. If the pressure stabilizes below
the high pressure alarm threshold before the 2 second delay is complete, the
alarm will not occur.

15
PumP overvIew
Common Delivery Specications
Upstream
occlusion sensor
•On
•O
Note: The upstream occlusion sensor is automatically disabled during use
with medication cassette reservoirs.
PCA Delivery Specications
Programming units If programming through manual mode. Otherwise the programming units are
preset through the CADD™-Solis Medication Safety Software.
•Milliliters (mL)
•Milligrams (mg)
•Micrograms (mcg)
Concentration mg/mL:
•0.1 to 0.5 mg/mL in increments of 0.1 mg/mL
•0.5 to 1 mg/mL in increments of 0.5 mg/mL
•1 to 15 mg/mL in increments of 1 mg/mL
•15 to 100 mg/mL in increments of 5 mg/mL
mcg/mL:
•1 to 15 mcg/mL in increments of 1 mcg/mL
•15 to 100 mcg/mL in increments of 5 mcg/mL
•100 to 500 mcg/mL in increments of 100mcg/mL
Continuous rate 0 to 100 mL/hr (or the mg or mcg equivalent)
PCA dose 0 mL to 50 mL (or the mg or mcg equivalent)
Max delivery rate (continuous rate + PCA dose): 40 to 250mL/hr
PCA dose lockout 1 minute to 24 hours in the following increments:
•1 minute for values between 1 and 20 minutes
•5 minutes between 20 minutes and 24 hours
Max doses per
hour
1 to 60
Delivery limit
amount
0.1 to 1900 mL (or the mg or mcg equivalent) in increments of:
•0.01 mL from 0.1 to 0.5 mL
•0.1 mL from 0.5 to 100 mL
•1 mL from 100 to 1,000 mL
•10 mL from 1,000 to 1,900 mL
Clinician bolus 0 to 50 mL (or mg or mcg equivalent)
Delivery limit
method
•Delivery limit
•Max doses per hour
•Not in use
Delivery limit
period
1 to 12 hours in increments of 1 hour
Max delivery rate,
combined bolus
and continuous
rate
40 to 250 mL/hr in increments of 1 mL
KVO rate •0 mL/hr
•0.1 mL/hr
Continuous Delivery Specications
Continuous rate 0.1 to 500 mL/hr
KVO rate 0.1 to 10 mL/hr
Intermittent Delivery Specications
Dose volume 0.1 to 1000 mL
Dose duration 1 min to 24 hr
16
PumP overvIew
Intermittent Delivery Specications
Dose cycle 10 min to 96 hr
Next dose start
time
0 to 96 hr in 5 min increments
KVO rate 0 to 10 mL/hr
Step Delivery Specications
Initial rate 0.4 to 499 mL/hr
Plateau rate 0.4 to 500 mL/hr
Rate increment 0.4 to 499 mL/hr
Infusion volume 1 to 9990 mL
Step duration 15 min to 24 hr
KVO rate 0 to 10 mL/hr
Step infusion alerts •On
•O
Taper Delivery Specications
Infusion volume 1 to 9990 mL
Taper up 0 min to 99:40 hr:min
Taper down 0 min to 99:40 hr:min
Infusion duration 10 min to 99:50 hr:min
KVO rate 0 to 10 mL/hr
Plateau rate upper
limit
0.1 to 500 mL/hr
CADD™ Ambulatory Tubing Set Testing
One representative medication for each of the following routes of delivery was tested for drug
interaction with pump disposables. Use any selected drug in accordance with the indications included
in the drug package insert. Administration of any drug by the CADD®-Solis VIP ambulatory infusion
pump is limited by any warnings, precautions, or contraindications in the drug labeling.
Route of Delivery Drug Tested
Intravenous, subarachnoid space (intrathecal) Morphine Sulfate Injection
Intra-arterial Floxuridine for Injection, USP
Intraperitoneal Dianeal with dextrose
Epidural space, local inltration
(subcutaneous, perineural, surgical site)
Ropivacaine HCl Injection
Remote Dose Cord
Smiths Medical provides a remote dose cord for use by the patient. e push button is a single pole double
throw (SPDT) switch. When the remote dose cord is attached to the pump, the patient may press the
remote dose cord button to receive a PCA dose. For easy access, the remote dose cord may be fastened to
the patient’s clothing or bedsheet with the attached clip.
NOTE: To detach the remote dose cord from the pump, grasp the remote dose cord connector and pull
straight back using a straight, steady motion.
CAUTION: Do not twist or turn the remote dose cord connector, or use any instrument to remove it from
the pump.
For additional specications, refer to the instructions for use provided with the product.

17
BatterIes
Batteries
Recommended Batteries
AA 1.5 volt primary (non-rechargeable) alkaline batteries (for example, Duracell®PC1500 / MN1500,
IECLR6) or the CADD®-Solis rechargeable battery pack are recommended for use in the CADD®-Solis
VIP ambulatory infusion pump.
Note: Smiths Medical does not recommend mixing new and used batteries; doing so may aect low
battery alarm times. Always select four new batteries when replacing depleted batteries.
CAUTION: Inspect the AA batteries for damage or wear to the metal or plastic insulation prior to use, or
after the pump has been dropped or hit. Replace the batteries if any damage is noted.
Replacing the Battery Door
If the battery door is removed or needs replacing, simply snap the door onto the
bar that is located on the pump.
CADD®-Solis Rechargeable Battery Pack
e battery pack is made up of a lithium-ion cell. When fully charged, its capacity is 5.2 Wh.
Each battery pack can sustain a minimum of 500 charge/discharge cycles. Within the operating
temperature range of 2°C to 40°C (36°F to 104°F), the battery pack becomes fully charged in 4hours or less.
e battery pack can be recharged using the CADD®-Solis AC adapter. It can be plugged directly into the
AC adapter or it recharges in the CADD®-Solis VIP pump with an AC adapter attached.
NOTE: Periodically inspect the rechargeable battery pack for damage or wear to the metal or plastic
insulation. Discontinue use if any damage is noted.
See the instructions for use supplied with the rechargeable battery pack for more information.
Battery Storage
e CADD®-Solis rechargeable battery pack should not be stored in a refrigerator. Recommended storage
conditions are 19°C to 25°C (66°F to 77°F).
Alkaline batteries should not be stored in a refrigerator. Recommended storage conditions are 10°C to
24°C (50°F to 75°F) with no more than 65% non-condensing relative humidity.
Battery power is quickly depleted at temperatures below 10°C (50°F). Aer 4 years of storage at 21°C
(70°F), an alkaline battery retains approximately 86% of its original capacity. Battery life is shorter if
the battery is stored above room temperature. An alkaline battery stored at 43°C (110°F) discharges to
approximately 80% of its capacity within one year.
Battery Life
Battery life is dependent on the following factors:
•Programmed delivery rate
•Operating temperatures
•Frequency of use and intensity of display backlighting
•Duration of use of the USB connector
•Battery storage conditions
•Battery type and brand
•Battery age
18
BatterIes
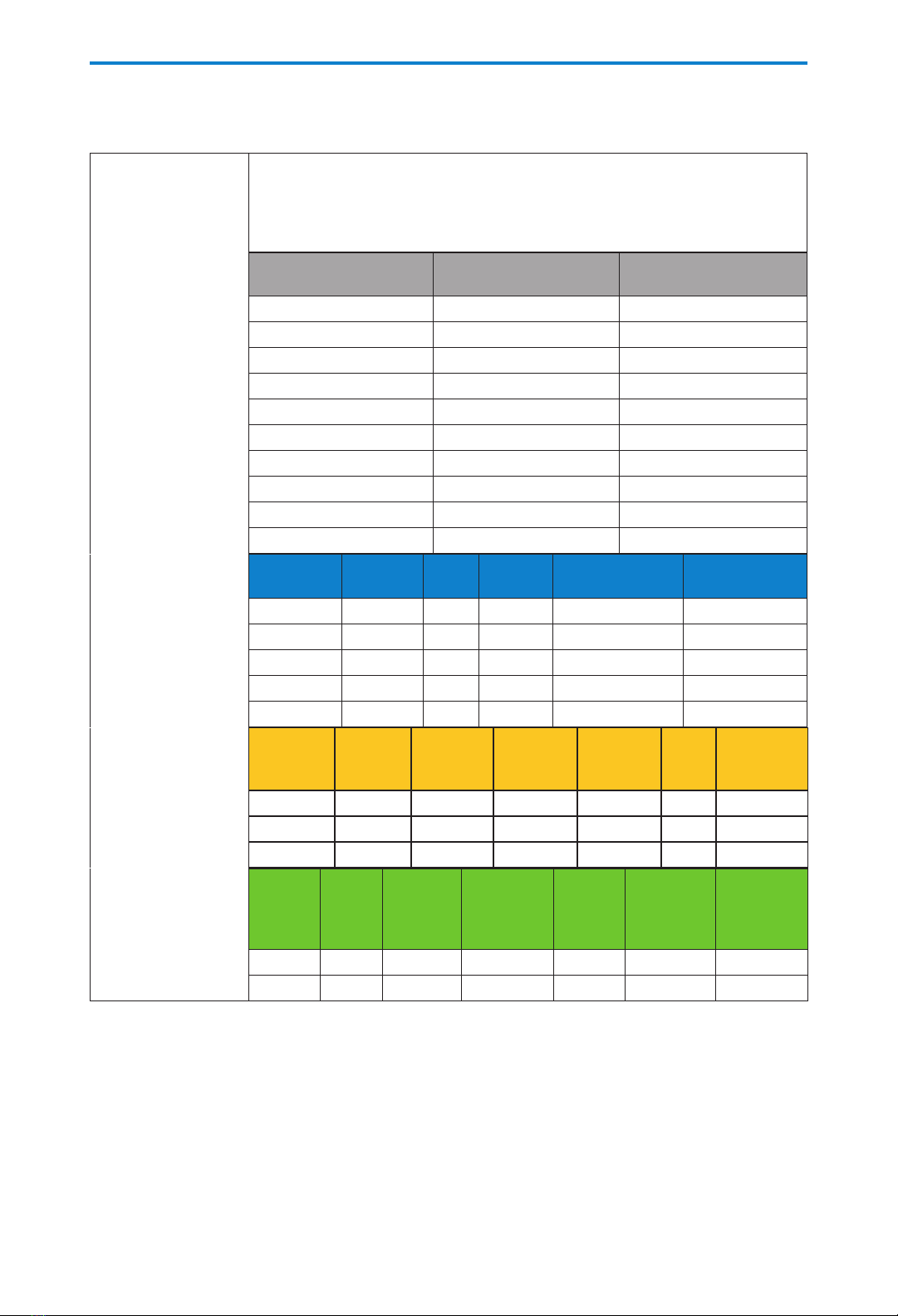
e following tables may be used to predict typical alkaline battery and CADD®-Solis rechargeable battery
pack life at dierent delivery rates. As expected, battery life decreases as the delivery rate increases.
Alkaline battery
life with screen
backlight intensity
set to 3
These estimates are based on laboratory tests conducted at room temperature
using new batteries (Duracell®PC1500 / MN 1500, IEC LR6). Actual battery life
varies depending on the battery brand, shelf life, temperature conditions,
delivery rate, and frequency of screen display and backlighting. It is
recommended that new batteries be kept available for replacement.
PCA and Continuous
delivery
(Max. delivery rate
= 100 mL/hr, when
using the PCA
delivery mode)
Delivery Rate
(mL/hr)
Operating Time
(hrs)
Vol. Delivered
(mL)
0.4 142 56
1.0 139 139
5.0 124 620
10.0 113 1130
30.0 69 2070
50.0 59 2950
125.0 37 4625
200.0 29 5800
350.0 15 5250
500.0 11 5500
Intermittent delivery Volume
(mL)
Duration
(hr)
Cycle
(hr)
KVO
(mL/hr)
Operating Time
(hrs)
Vol. Delivered
(mL)
20.2 1 4 0.2 131 684
40.7.0 1 4 0.2 116 1221
61 .0 1 6 0.2 111 1177
125 .0 1 6 0.2 75 1637
200 0112 0.2 111 2020
Step delivery Volume
to Deliver
(mL)
Starting
Rate
(mL/hr)
Step
Duration
(min)
Step Rate
Increase
(mL/hr)
Max Rate
(mL/hr)
No.
of
Steps
Operating
Time
(hrs)
900 45 15 45 315 723
1500 37. 5 30 80 300 524
2500 30 30 90 300 419
Taper delivery Volume
(mL)
Period
(hr)
Taper Up
(hr)
Taper
Down
(hr)
KVO
(mL/hr)
Operating
Time
(hrs)
Vol.
Delivered
(mL)
2000 12 1 1 5 31 5800
3000 12 1 1 5 23 6460
19
BatterIes
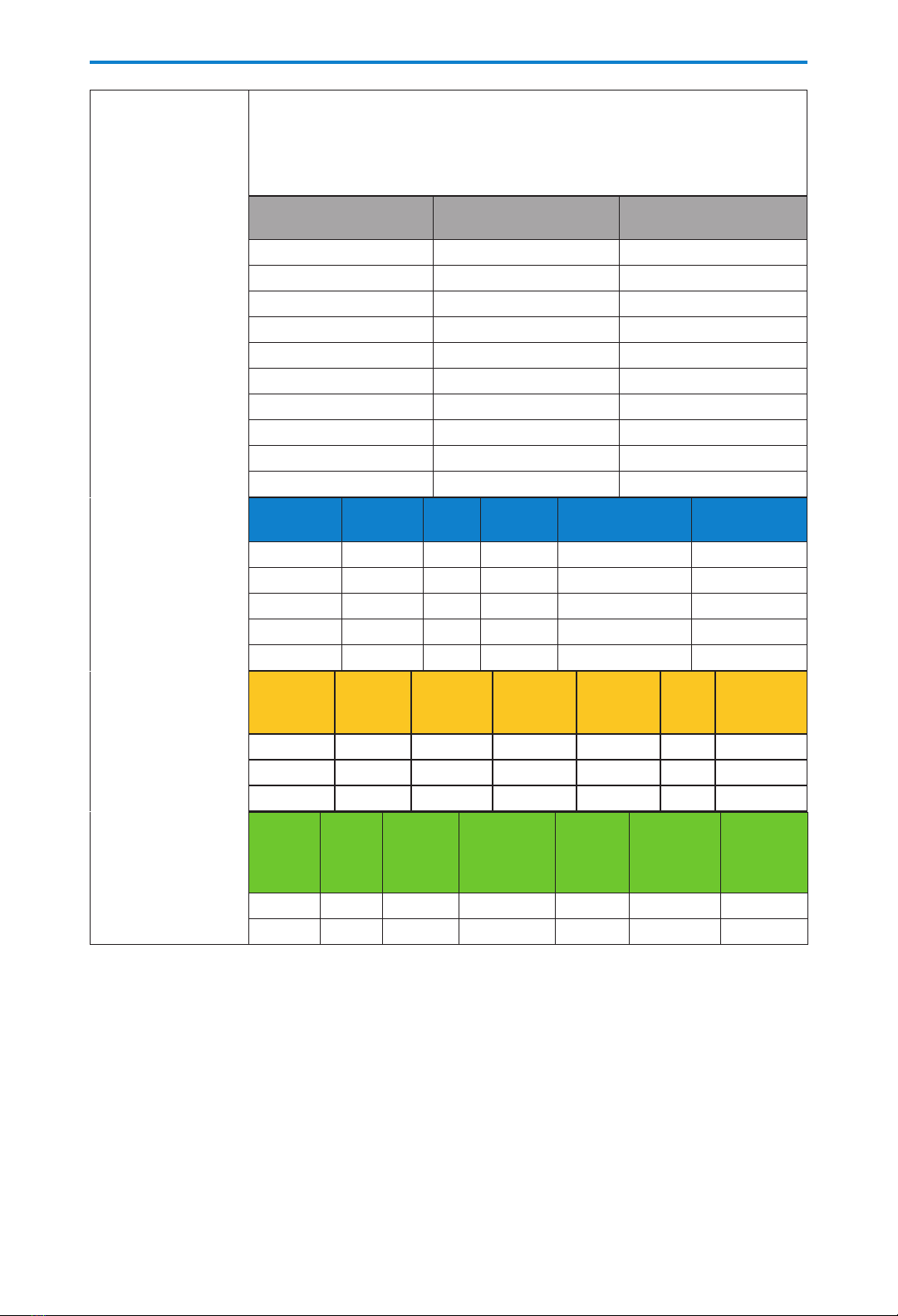
Rechargeable
battery pack
life with screen
backlight intensity
set to 3
These estimates are based on laboratory tests conducted at room temperature
using a new CADD®-Solis rechargeable battery pack. Actual battery life varies
depending on temperature conditions, delivery rate, and frequency of screen
display and backlighting. It is recommended that new batteries be kept
available for replacement.
PCA and Continuous
delivery
(Max. delivery rate
= 100 mL/hr, when
using the PCA
delivery mode)
Rate
(mL/hr)
Life
(hrs)
Volume
(mL)
0.4 74 29
1.0 67 67
5.0 60 300
10.0 50 500
30.0 40 1200
50.0 35 1750
125.0 30 3750
200.0 20 4000
350.0 13 4550
500.0 10 5000
Intermittent delivery Volume
(mL)
Duration
(hr)
Cycle
(hr)
KVO
(mL/hr)
Operating Time
(hrs)
Vol. Delivered
(mL)
20.2 1 4 0.2 81 436
40.7.0 1 4 0.2 68 702
61 .0 1 6 0.2 85 929
125 .0 1 6 0.2 53 1133
200 0112 0.2 95 2424
Step delivery Volume
to Deliver
(mL)
Starting
Rate
(mL/hr)
Step
Duration
(min)
Step Rate
Increase
(mL/hr)
Max Rate
(mL/hr)
No.
of
Steps
Operating
Time
(hrs)
900 45 15 45 315 717
1500 37. 5 30 80 300 517
2500 30 30 90 300 415
Taper delivery Volume
(mL)
Period
(hr)
Taper
Up
(hr)
Taper
Down
(hr)
KVO
(mL/hr)
Operating
Time
(hrs)
Vol.
Delivered
(mL)
2000 12 1 1 5 21 3830
3000 12 1 1 5 17 4640
20
BatterIes
Collect Separately
is product contains electrical and electronic components (including batteries) that may contain
materials, which if disposed of with general waste, could be damaging to the environment.
In accordance with Directive 2002/96/EC Waste Electrical and Electronic Equipment, residents of the
European Union must follow specic disposal or recycling instructions for this product. Contact your
local distributor, or visit the following web site for specic instructions:
http://www.smiths-medical.com/recycle/index.html.
Non-European Union residents must dispose of or recycle this product (including batteries) in accordance
with the local laws or regulations that apply.
WARNING: There are potential health hazards associated with improper disposal of batteries,
electronics, and contaminated (used) reservoirs and extension sets. Dispose of used batteries,
reservoirs, extension sets, and other used accessories, or a pump that has reached the end of its
useful life, in an environmentally safe manner, and according to any regulations that may apply.
Table of contents
Other Smiths Medical Water Pump manuals

Smiths Medical
Smiths Medical CADD Administration Sets with Flow Stop Reference guide

Smiths Medical
Smiths Medical CADD-Prizm PCS II User manual

Smiths Medical
Smiths Medical CADD-Solis User manual

Smiths Medical
Smiths Medical CADD Solis 2100 User manual

Smiths Medical
Smiths Medical Graseby 500 User manual

Smiths Medical
Smiths Medical CADD-Prizm VIP 6100 Reference guide

Smiths Medical
Smiths Medical CADD Prizm VIP 6100 User manual

Smiths Medical
Smiths Medical Graseby C9 User manual

Smiths Medical
Smiths Medical CADD Solis VIP 2120 User manual