SOMNI 3 User manual

VETERINARY ANESTHETIC
VAPORIZER OPERATIONS MANUAL
SOMNI 3
SOMNI PRODUCT MANUAL

TABLE OF CONTENTS
User Responsibility
Warnings and Cautions
Principles of Operation
Examination and
Preparation For Use
Installation
Operating Instructions
Filling Instructions
Funnel Fill
Draining Instructions
Funnel Fill
Specications
Warranty and Service
2
3
4
5
6
7
9
11
12
14
17

USER RESPONSIBILITY
The SOMNI 3 is a precision medical vaporizer designed for Veterinary
Use Only. This vaporizer was designed to mix the vapors of specied
liquid anesthetic gas agents with anesthetic delivery gases. The vaporizer
will continue to provide reliable performance only if the manufacturer’s
operating and maintenance instructions are followed. The vaporizer,
as with any mechanical medical device, requires periodic preventative
maintenance and calibration. Any components, which become worn,
distorted, or contaminated should be replaced by a factory authorized
service center. A vaporizer that requires service should not be used until
it has been accurately tested and veried by a factory authorized service
center. Field-testing of vaporizer output using portable test equipment,
while valuable, is not a substitute for factory authorized preventative
maintenance. Factory service and calibration is recommended every
two years, eld testing is recommended annually at minimum. The
user assumes full responsibility and liability of any use of the vaporizer.
3

WARNINGS AND CAUTIONS
• This vaporizer is intended for Veterinary Use Only
• Do not ll the vaporizer with any agent other than the one specied on the front
label. Vaporizers are specically designed dependent on agent type. Therefore,
any other agent used can prove to be dangerous to a patient.
• Do not use this vaporizer until it is mounted upright, vertical and out of operation
for a minimum of (1) hour aer initial lling to allow for proper initial wick
absorption. Failure to allow wicking time may result with inaccurate output
at the selected dial setting.
• Do not carry vaporizer by control dial. Handle and transport vaporizer with care
by grasping the vaporizer rmly with two hands.
• Do not modify, tamper, or disassemble the vaporizer. There is a probable
danger of damaging the vaporizer and altering the calibration accuracy.
• Do not put vaporizer into any liquid, including water.
• Do not attempt to sterilize vaporizer.
• Do not drain anesthetic agent into any container other than a properly
marked container.
• Do not tilt or tip vaporizer beyond a 45-degree angle while lled with liquid
agent. If unit is accidentally tipped on its side, please call the manufacturer
for specic instructions.
• Do not have vaporizer serviced by anyone other than a SOMNI Scientic
authorized service center.
• Do not operate vaporizer prior to leak testing the anesthetic equipment,
ensuring secure connections to prevent unnecessary anesthetic exposure
• Turn the vaporizer OFF when not in use
• It is recommended that the vaporizer is kept upright at all times, aer installation.
4
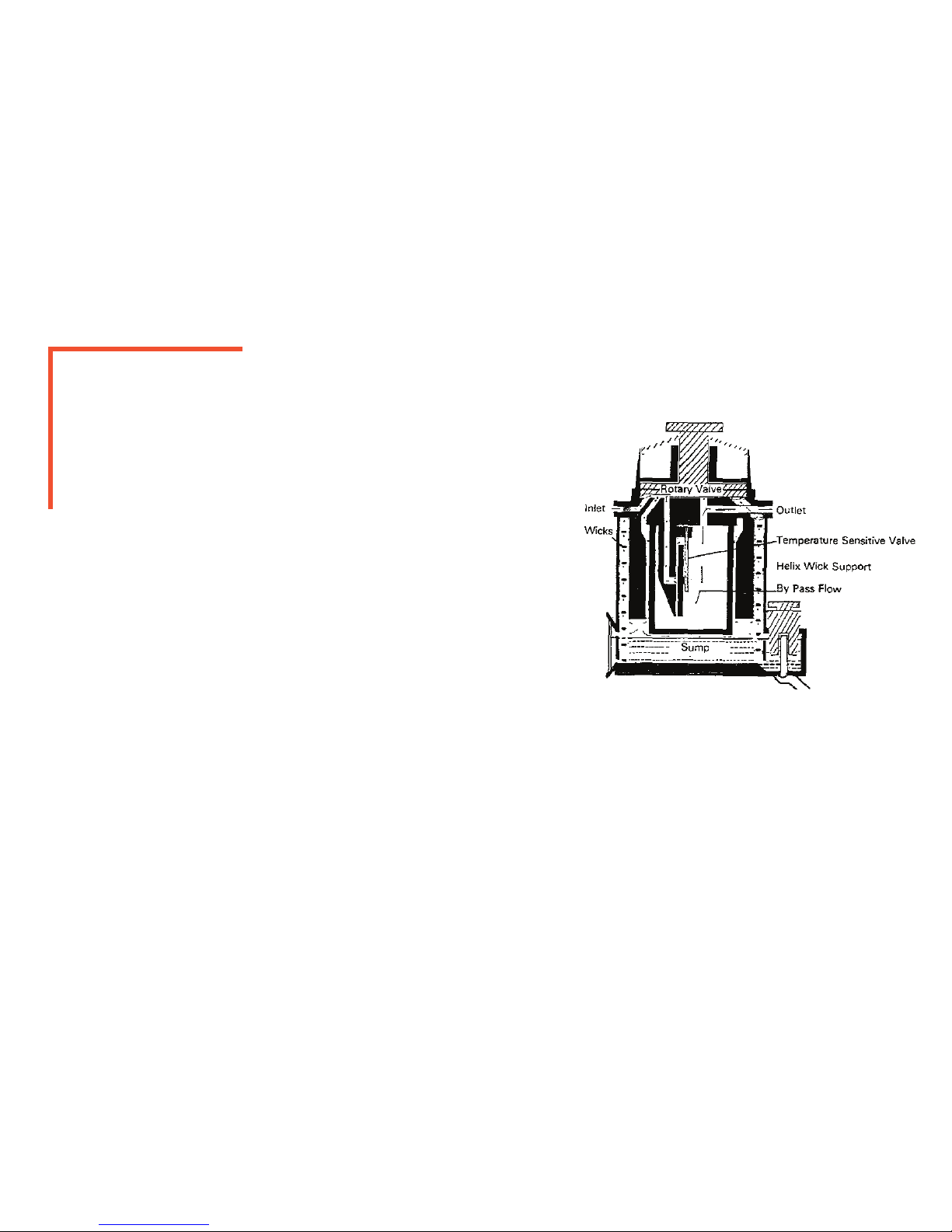
PRINCIPLES OF OPERATION
VAPORIZER SUMP AND VALVE ASSEMBLY
5
This anesthetic vaporizer is comprised of a vaporizing chamber and duct
system (located within the sump cover), rotary valve and concentration dial. The
concentration dial is connected to the rotary valve underneath. The rotary valve
contains ducts and a vapor control channel. With the concentration dial in the
o position, the rotary valve links the inlet and outlet of the vaporizer, allowing
carrier gas to pass through. When the concentration dial is turned on, the carrier
gas is split in, a stream and a stream owing into the vaporizing chamber.
The vaporizing chamber contains two concentric wicks that are in contact
with the liquid anesthetic agent. The wicks ensure the vapor is maintained at sat-
uration of concentration in the gas that leaves the vaporizing chamber. The ow
through the vaporizing chamber is controlled by the concentration dial.
Temperature compensation occurs automatically utilizing a bi-metallic
strip to keep the output of the vaporizer constant during conditions of
changing temperature.
Concentration Setting Dial
Filler

EXAMINATION AND PREPARATION FOR USE
1. Examine shipping carton for signs of external damage.
2. Remove contents from carton and inspect for visible damage
such as dents or missing parts.
3. If damage or missing parts are discovered or suspected, notify
customer service immediately at 1-877-637-3625.
4. Check that control dial operates freely.
5. Conrm the drain valve is completely closed .
6. Remove the mounting hardware (vaporizer spacer and 3-6mm bolts with
washers) and drain tube (*not shown) from package.
6

INSTALLATION
The standard mounting system requires bolting of the vaporizer directly
to a rigid back bar of an anesthetic gas machine. The vaporizer should
always be mounted between the gas ow-metering unit and the
breathing circuit-always upstream of any absorber or humidier.
Ensure that emergency oxygen supplies or oxygen ush enter the
gas circuits downstream of the vaporizer.
The SOMNI 3 is supplied with mounting hardware consisting
of a spacer and 3-6mm bolts. While supporting the weight of
the vaporizer, install the spacer between the vaporizer and the
mounting surface and secure the vaporizer using the mounting
bolts provided. Ensure the bolts are tightly secured, but be sure
not to over-tighten.
Connect the 23mm inlet and outlet adapters to the corresponding
outlets on the cagemount. Twist and rmly push on for a secure,
leak-free connection. It may be necessary to connect the 23mm
adapters prior to mounting the vaporizer.
Complete a 10 second pressure test to conrm a leak free
installation. Instructions on Page 10.
7
A.
B.
C.

INSTALLATION
8
• The direction of gas ow must be from “inlet to outlet”
(i.e. from le to right) when viewing the vaporizer from
the front.
• Ensure the liquid (which may accumulate in the
breathing circuit or the CO2 absorber) can not enter
the vaporizer while:
The vaporizer is tted with standard 23 mm inlet and
outlet taper connections.
IMPORTANT! Vaporizer Spacer Placement
-in use
-during disassembly of the circuit
-when the machine is not in use

OPERATING INSTRUCTIONS
1. Observe all instructions and warnings on the vaporizer
2. Fill only with agent indicated with vaporizer in OFF position
3. Perform a leak test prior to rst use (See instructions
below- “10 Second Test”)
4. Depress locking button to turn dial from OFF position
5. Turn dial counterclockwise to desired concentration
6. Then turn control dial counter clockwise to desired concentration
7. Turn vaporizer to “OFF” position when not in use
9

10 SECOND PRESSURE TEST
10
Before each use, “leak test” the anesthesia system and ensure the waste gases have a
patent way through the evacuation system.
www.somniscientific.com | info@somniscientific.com | 1-877-637-3625
Close the pop-o valve and create a tight seal of the patient circuit
with your hand.
Push the oxygen flush button, or turn on oxygen flow meter, until the
bag is fully distended and/or manometer reads 30-40 cm of H20
pressure.
Release the oxygen flush button and/or ensure the oxygen flowmeter
is o; float is at the bottom of the tube. Observe the manometer (or
the bag if your machine does not have a manometer). The manometer
needle should remain steady. If the manometer needle drops, the bag
deflates, or if you hear a hissing sound, you have a leak.
Check hoses, bag, vaporizer inlet and outlet, any fittings or seals at the
top and/or bottom of your CO2 absorbent container for leaks (a soapy
water solution can be used for leak test). When the pressure remains
constant, with the oxygen turned o, your machine can be considered
leak-free on the low-pressure side.
Re-open the pop-o to your standard setting.
Squeeze the bag (with your hand still over the patient circuit) to ensure
the gases have a clear way out through your scavenging system.
Before each use, “leak test” the
machine, and also ensure that the waste
gases have a clear way through your
evacuation system.
1
2
3
4
5
6

11
FILLING INSTRUCTIONS-FUNNEL FILL
DO NOT ll vaporizer with any agent other than the one specied
on the front label. The vaporizer is designed for that agent only. Any
agent other than specied could prove to be dangerous to a patient.
WARNING:
1. Verify that vaporizer dial and delivery gas owmeter(s) are
in the “OFF” position.
2. Verify that anesthetic agent is the same as labeled on front
of vaporizer.
3. Verify that the drain valve on the right side of the ll assembly
is closed by turning clockwise until nger tight.
4. Remove the funnel cap and pour agent slowly into opening.
Simultaneously, observe the agent level through the sight
glass. Note: If the vaporizer is dry, the level will fall slightly
as the wick absorbs the agent.
5. Replace cap by turning cap clockwise. Cap should be tight
to prevent leaks.
Drain Valve
Drain Port
Fill Cap

12
DRAINING INSTRUCTIONS-FUNNEL FILL
Liquid MUST be drained from the vaporizer into a properly labeled container.
WARNING:
1. Attach small drain tube to the hole in the front of the vaporizer
ll assembly.
2. Hold an empty, properly labeled container under the tube.
3. Open the drain valve located on the right side of the ll assembly.
Aer all liquid is drained, close the valve nger tight to seal.
4. Discard used anesthetic agent per proper protocol.

ASSURING PERFORMANCE OF YOUR VAPORIZER
To assure the continued performance of your vaporizer, the manufacturer
recommends full factory preventative maintenance be performed
every 2 years. Accurate and eicient anesthetic gas delivery is a primary
consideration in patient care. Anesthetic agent vapors are extremely
potent, and a very small error in concentration could be hazardous.
Preventative Maintenance Includes:
• Disassembly of the vaporizer
• Cleaning and inspection of all components
• Replacement of internal wick and seals
• Testing of thermostat and adjusting or replacing if needed
• Reassembly and leak test
• Calibration using industry standard laser refractometer
Preventative Maintenance Ensures:
o Worn components are replaced when necessary and
calibration is veried.
o Wicks are replaced to prevent the accumulation of contaminants
which can hinder anesthetic vaporization and interfere with eicient
anesthetic gas delivery.
o Inspection and service can reveal accidental damage that could alter
performance and allows correction
o Correction of vaporizer leaks and prevents the vaporizer from
contributing to unnecessary personnel exposure to waste anesthetic
gas pollution.
13

Calibration
Vaporizers are calibrated at 21° C. The variation in output
with temperature, ow rate and duration of use is small, and
the variation in output when used with Intermittent Positive
Pressure Respiration is negligible.
Resistance to Gas Flow
5cm.wg at the “OFF” setting at 5 liter/min 02 at 22° C
Duration of Use
The rate of consumption of anesthetic agent depends
primarily on ow rate and vapor output concentration. As
an approximate working gure, 1.0 ml of liquid anesthetic is
required to provide 200 ml of vapor.
The rate of evaporation of anesthetic agent may be used
(with caution) as an approximate method of checking that
the delivered output is not grossly in error. It may also be
used as a means of estimating how oen the vaporizer is
likely to need relling.
The approximate hourly consumption of anesthetic agents
can be expressed as follows:
3 x % x F
Where % represents the setting of the vaporizer output
percentage, F represents the input ow rate in liter/min.
Example: If a vaporizer is set to deliver 2% at 6 liter /min
total input gas ow rate.
SPECIFICATIONS
Approximate rate of agent consumption = 3 x 2 x 6 = 36 ml/hour.
The above gures are approximate and intended for clinical
guidance only. Figures will vary depending on ow meter type
(and other varying factors). Results will be grossly in error if the
vaporizer drain port is not fully closed.
Liquid Capacity
Amount of anesthetic agent to fully charge the vaporizer =250 ml.
Amount retained by Wick System =75 ml.
Weight and Dimensions
Weight 15.5 lb. / 7kg
Height 7.58 in / 195 mm
Depth 5.5 in / 140 mm
Width 5.25 in / 135 mm
Capacity 250 ml
Wicking Capacity 75 ml
Some ventilators may impose higher, steady back pressures
(around 100 mm HG), producing more signicant depression of
the v/v percentage. Increased patient uptake of agent, along with
improved ventilation can oen mitigate these eects, eliminating
the need to compensate for increased back pressure at the
vaporizer.
High Back Pressures;
Pressures in excess of 400mm Hg could conceivably occur during
procedures similar to bronchoscopy or because of occlusion of
downstream tubing and piping or for other reasons. These eects
on v/v percentage cannot be precisely predicted but the most likely
eects will be reductions in concentration (or small increases).
14

Back Pressure Fluctuating
Fluctuating backpressures may be imposed on the
vaporizer by downstream components and assisted
or controlled ventilation to the patient. This can
aect the vaporizer and increase the concentration by
intermittently altering the pressures, therefore altering
ow distribution within the vaporizer. The greatest
eects are observed in combinations of very low ow
rates and low dial settings, with large and rapid pressure
uctuations. These eects become progressively less
notable as the dial setting and ow rates increase,
causing the magnitude and rate of cycling pressure
uctuations to decrease.
In clinical use, vaporizers are considered unaected
by uctuating back pressures which occur frequently
in most typical, clinically encountered conditions
appertaining to human anesthesia.
Carrier Gas Composition
Small eects can occur when the carrier gas
composition is changed (i.e., from Oxygen to air, or in a
Nitrous Oxide and Oxygen mixture). As a general rule,
variation of output with carrier gas compilation should
be considered of low clinical signgance, since the side
eects (if any) are typically less than 10% of the setting.
SPECIFICATIONS
In an instance where signicant changes do occur, the usual
eect is a slightly depressed output once nitrous oxide is
employed (compared to the output when oxygen is the carrier
gas).
Other Variables
Ambient temperature, input ow rate and duration can oen
aect delivered concentrations, particularly when vaporizers
are used at the clinical extremes of such variables.
The valve design and temperature compensation system of
the Somni 3 vaporizers work to reduce the eects to levels
considered not signicant by clinical standards.
15
SPECIFICATIONS
16
Eects of Variables
Temperature
Temperature variation eects are typically negligible
at common dial setting and ambient temperature
combinations.
The vaporizer responds very slowly to change in
ambient temperate to prevent the valve from closing
completely. As an additional safety feature, the
temperature sensitive valve does not respond to
temperatures below the approximate range of 12-15° C.
Should the vaporizer temperature be lower than this,
then the output can be expected to be lower than that
indicated on the dial.
At temperatures above the range shown on the
performance curves, the vaporizer output may be
unpredictably high-particularly if the temperature
approaches the boiling point of the anesthetic agent.
To avoid any inaccuracies due to extreme temperatures,
the vaporizer should be allowed to reach a temperature
within the suggested range (of the performance curves)
prior to use.
Pressure
Vaporizers are graduated in v/v percentage at 760 mm Hg.
If the ambient pressure changes the v/v % will change so
that at an ambient pressure P mm Hg the delivered
percentage (D % v/v)-
Equation 1 D = % x 760
Where % is the nominal setting of the vaporizer.
It is generally accepted that the depth of anesthesia depends
on the inspired partial pressure of agent and not the
concentration by volume of agent.
To obtain a consistent depth of anesthesia when gross
changes of barometric pressure occur, it is necessary to
change the v/v percentage in inverse proportion to the
barometric pressure. The vaporizer automatically performs
this action. For practical and clinical purposes, the eects of
barometric pressure can be ignored.
The vaporizer automatically does this and for practical clinical
purposes the eects of the barometric pressure
can be ignored.
P

1-877-637-3625 info@somniscientic.com www.somniscientic.com
17
Limited Warranty
SOMNI Scientic (SOMNI) warrants to the original purchaser
that the products, not including accessories, shall be free
from defects in materials and workmanship under normal
use, if maintained in accordance with SOMNI’s guidelines
and used according to its labeling, for the period specied
in the manual.
Warranty period is Lifetime contingent upon a 2 year service
cycle from the invoiced date of purchase.
THIS LIMITED WARRANTY, IS IN LIEU OF AND EXCLUDES ALL
OTHER WARRANTIES WHETHER EXPRESSED OR IMPLIED,
BY OPERATION OF LAW OR OTHERWISE, INCLUDING
BUT NOT LIMITED TO, ANY IMPLIED WARRANTIES OF
MERCHANTABILITY OR FITNESS FOR A PARTICULAR
PURPOSE.
This warranty is void if the product has been altered,
misused, damaged by neglect or accident, tampered with,
not properly maintained, not installed in strict compliance
with applicable codes and ordinances, or repaired by
persons not authorized by SOMNI. This warranty does
not cover normal wear and tear and maintenance items
and specically excludes accessory items and any other
equipment used with the product.
WARRANTY AND SERVICE
Limitation of Remedies
SOMNI Scientic’s only obligation under this limited warranty is
the repair or replacement of the product. THIS IS THE EXCLUSIVE
REMEDY. SOMNI shall not be liable for and hereby disclaims any
direct, incidental, consequential or special damages or delays,
including but not limited to loss of use, downtime, lost business,
revenues and prots.
Warranty Procedure
To obtain warranty service, contact SOMNI Scientic
877-637-3625 or info@somniscientic.com.
SOMNI Scientic
1900 Sleepy Hollow Road,
South Park, PA 15129
Table of contents
Other SOMNI Vaporizer manuals


















