Instructions
Intended Use
This device is intended for medical purposes to illuminate body surfaces. It is
used for non-invasive visual examination of intact skin.
This battery-operated product is designed for external examination only within
professional healthcare facilities by medical professionals.
Check the correct operation of the device before use! Do not use the device if
there are visible signs of damage.
CAUTION: Do not look directly into the LED light. Patients must close their
eyes during examinations.
In case of a serious incident with the use of this device, notify 3Gen immediate-
ly and, if required by local regulations, your national health authority.
CAUTION: Do not use the device in re or explosive risk area (e.g. oxygen-rich
environment).
WARNING: This product can expose you to chemicals including methylene
chloride and hexavalent chromium, which are known to the State of California
to cause cancer or reproductive toxicity. For more information go to www.
P65Warnings.ca.gov.
Electromagnetic Compatibility
This device complies with the EMC Emissions and Immunity level requirements
of the standard IEC 60601-1-2:2014. The emissions characteristics of this
equipment make it suitable for use in industrial areas and hospitals (CISPR 11
class A). If it is used in a residential environment (for which CISPR 11 class
B is normally required), this equipment might not oer adequate protection
to radio frequency communication services, and the user might need to take
mitigation measures, such as relocating or re-orienting the equipment.
WARNING: Use of this equipment adjacent to or stacked with other equipment
should be avoided because it could result in improper operation. If such use
is necessary, this equipment and the other equipment should be observed to
verify that they are operating normally.
WARNING: Use of accessories other than those provided by the manufactur-
er of this equipment could result in increased electromagnetic emissions or
decreased electromagnetic immunity of this equipment and result in improper
operation.
WARNING: Portable RF transmitters should be used no closer than 30 cm (12
inches) to any part of the device. Otherwise, degradation of the performance of
this equipment could result.
WARNING: To avoid the risk of electrical shock, inspect the battery charger
cable and plug for damage before use. Do not use the v900L or accessories if
damaged.
CAUTION: DO NOT drop any component of the system. Physical shock may
cause permanent damage.
CAUTION: DO NOT use the system as a light to navigate your environment.
CAUTION: DO NOT allow metal objects or body parts to touch electrical
connections.
CAUTION: DO NOT operate machinery, equipment or vehicles with the v900L
positioned on the head and do not walk or climb stairs while wearing the v900L
to avoid a tripping or falling accident.
CAUTION: DO NOT wear the v900L if you experience any strain in the neck,
eyes or other areas. Turn v900L o before storage.
CAUTION: DO NOT block any opening of the system. Doing so may cause
overheating of the Illuminator Module. Avoid operating the system in dusty
environments.
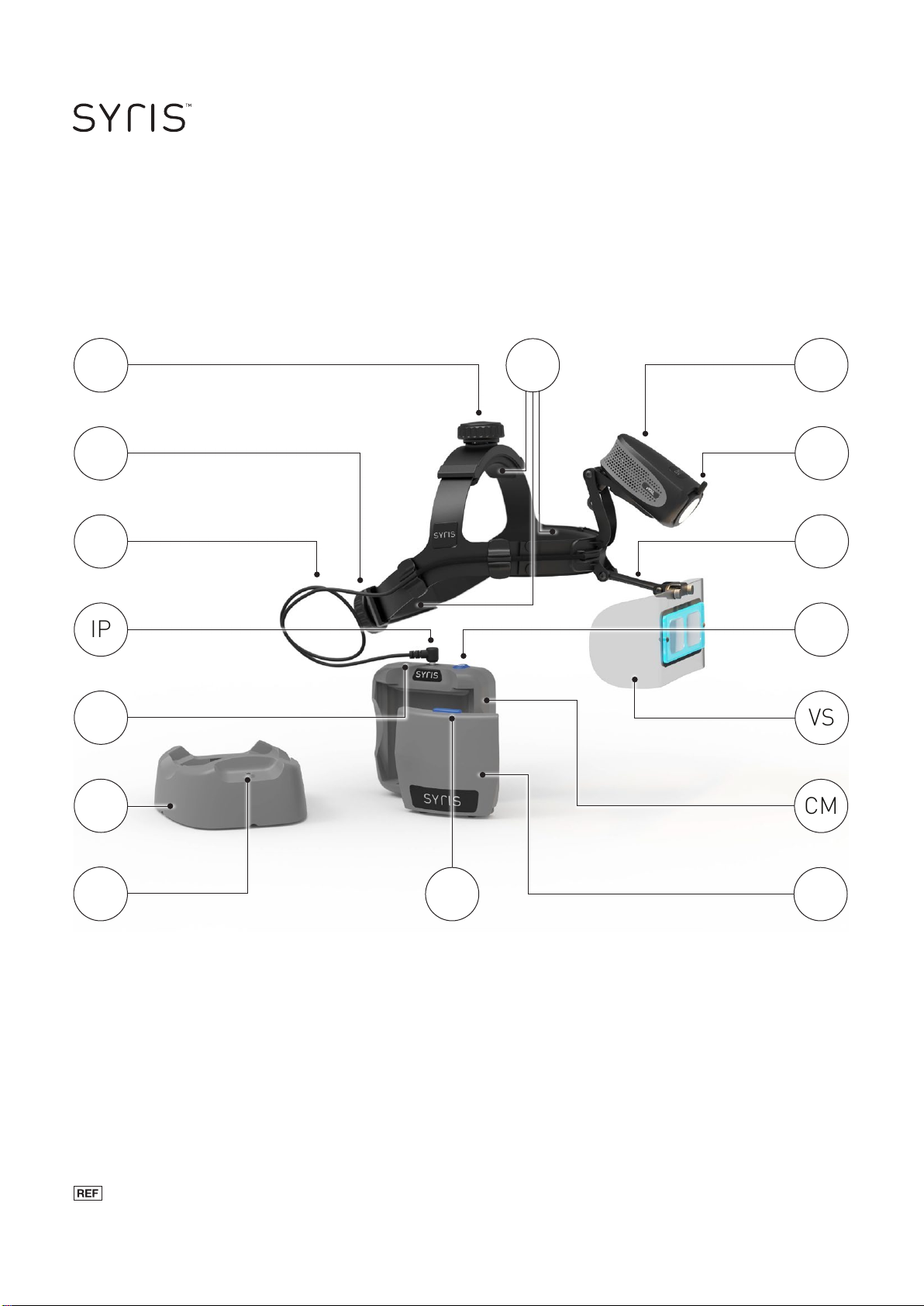
The Syris v900L Vision Enhancement System combines proprietary LED
illumination, polarization, and magnication technology to enhance the vision of
medical professionals during dermatological procedures. The system removes
surface glare from the patient’s dermal layer promoting improved viewing of
sub-surface features.
Powered by a lithium ion battery, the system provides mobility for the user. The
polarizer rotates to allow for surface and sub-surface viewing. The adjustable
Visi-Shield provides magnication and a clear eld of view.
CAUTION: DO NOT position the AC power supply and Charger Base (CB)
where it is dicult to disconnect the power supply from the AC wall outlet.
CAUTION: DO NOT plug the power supply’s L-shaped connector into Control
Module (CM).
CAUTION: Do not use a battery (B) that is visibly damaged or the Charger
Base indicates a faulty battery.
CAUTION: This device uses a special 7.4V lithium-ion battery which can only
be purchased from 3Gen or an authorized 3Gen dealer. Do not under any
circumstances use another battery other than the one designed for this unit.
Before initial use of the v900L, you should fully charge the battery (B) using the
Charger Base (CB).
Select the appropriate AC adapter for your region and ensure that it snaps
securely into the power supply. Plug the power supply into an approved wall
outlet (100-240V, 50/60Hz and its small right-angle plug into the Charger Base.
Without a battery installed, the Charge Indicator (CI) is solid red.
Install the Battery into the Charger Base by placing its bottom edge on the
Base’s ledge, then pressing the Release Button (RB) and pushing the battery
into the Charger Base until it snaps in place. The Charge Indicator (CI) LED
begins to blink green indicating that charging has begun. Charging is complete
when the LED turns solid green, approximately ve hours for the initial full
charge. A blinking red LED indicates a faulty battery or charger. Removing the
battery requires pressing the Release Button (RB).
To allow for nearly uninterrupted use of the v900L, it is recommended that
multiple batteries are purchased (part no. VTS1000-BAT).
Using the Control Module
CAUTION: DO NOT touch the battery contact of the Battery (B) and the patient
at the same time.
With the battery inserted into the Control Module (CM), attach it to your belt.
Prior to inserting the L-shaped plug of the Illuminator Cable (IC) into the Con-
trol Module, make sure your belt is secure on the body.
Press the blue power button (PB) on the Control Module for full brightness.
Depressing it a second time reduces the light intensity. Depressing it a third
time shuts o the power.
The Control Module is equipped with a three-level Battery Indicator (BI). The
indicator is green when over 30 minutes of operation time remain; it turns or-
ange at less than 30 minutes and red when less than 10 minutes at full power
remain. When red, the unit emits three audible signals every 30 seconds until it
automatically shuts down. Actual times may vary.
Operating at the lower brightness setting extends the operation time by approx-
imately 33%.
Wearing the Device
After placing the v900L device on your head, a secure and comfortable t is
achieved with the use of two adjustment knobs. To adjust elevation, use the
Top Knob (TK). For a tighter t, turn the Back Knob (BK) clockwise. Turning
the knob counter-clockwise achieves a looser t. Position the v900L on your
head and turn the Top Knob (TK) to ensure that the front of the headband
sits comfortably above your eyebrows and the rear of the headband sits at
the base of the occipital lobe. Use the Back Knob (BK) for comfortably tight t
around your head.
Adjust the position of the Visi-Shield (VS) by gently raising or lowering its
Support Arm (SA) and moving the Visi-Shield forward or back. When properly
positioned, the magnifying lens should be in your normal line of sight.
Move the Illuminator Module (IM) up or down to illuminate your exam eld. Turn
the Polarizer (P) to enhance sub-surface or surface structures, as desired.
With a charged battery installed in the Control Module (CM) and the Illuminator
Cable (IC) connected to the Control Module, press the blue Power Button (PB)
to turn on the V900L at its brightest setting. An audible beep and a green LED
conrm that the unit is on. To reduce light intensity, push the button a second
time, conrmed by an audible beep. A third press of the button turns the unit
o, which is conrmed by another audible beep and all LEDs turning o.
Headband Pads
The Headband Pads (HP) may be cleaned or replaced as desired. To maintain
a high level of comfort and sanitation, it is recommended that the pads be
replaced monthly. Additional Headband Pads (part no. v900L-80) may be
purchased from 3Gen or your 3Gen dealer.
Troubleshooting
Please check www.dermlite.com for the most current troubleshooting informa-
tion. If your device requires servicing, visit www.dermlite.com/service or contact
your local 3Gen dealer.
Care and Maintenance
CAUTION: No modication of this equipment is allowed.
Your device is designed for trouble-free operation. Repairs shall be made only
by qualied service personnel.
Cleaning
The exterior of your device, except the optical parts, may be wiped clean
with isopropyl alcohol (70% vol.) prior to use on a patient. The lens should be
treated as high-quality photographic equipment and should be cleaned with
standard lens cleaning equipment and protected from harmful chemicals. Do
not use abrasive material on any part of the equipment or immerse the device
in liquid. Do not autoclave.
Warranty: 10 years for parts and labor. The battery is warranted for 1 year.
Disposal
This device contains electronics and a lithium-ion battery that must be sepa-
rated for disposal and may not be disposed of with general household waste.
Please observe local disposal regulations.
The Syris v900L set includes: V900L vision system (illumination module
with adjustable polarization, 3 removable headband pads, illuminator cable,
Visi-Shield with polarizer and magnifying lens), control module, charger base,
battery, power supply with international AC adapters, storage case
Technical Description
Visit www.dermlite.com/technical or contact your local 3Gen dealer.
ENGLISH
©2021 by 3Gen Inc. | 06 May 2021 | V900L-1701A
(English)