2TAKARA BIO INC.
CycleavePCRTM O157 (VT1/VT2) Detection Kit Ver.2.0 v.0607
Cat.#CY203
URL:http://www.takara-bio.com
I. Description: Enterohemorrhagic E. coli (EHEC) such as O157:H7 is a class of pathogenic
E. coli
which
causes hemorrhagic colitis with accompanying melena and severe abdominal pain, and in
addition, hemolytic uremic syndrome. These serious symptoms are caused by verotoxin, a
cytotoxin produced by EHEC. It has been pointed out that in detection of EHEC, an assay
method of accurately and promptly determining if there are these verotoxin genes or not is
important.
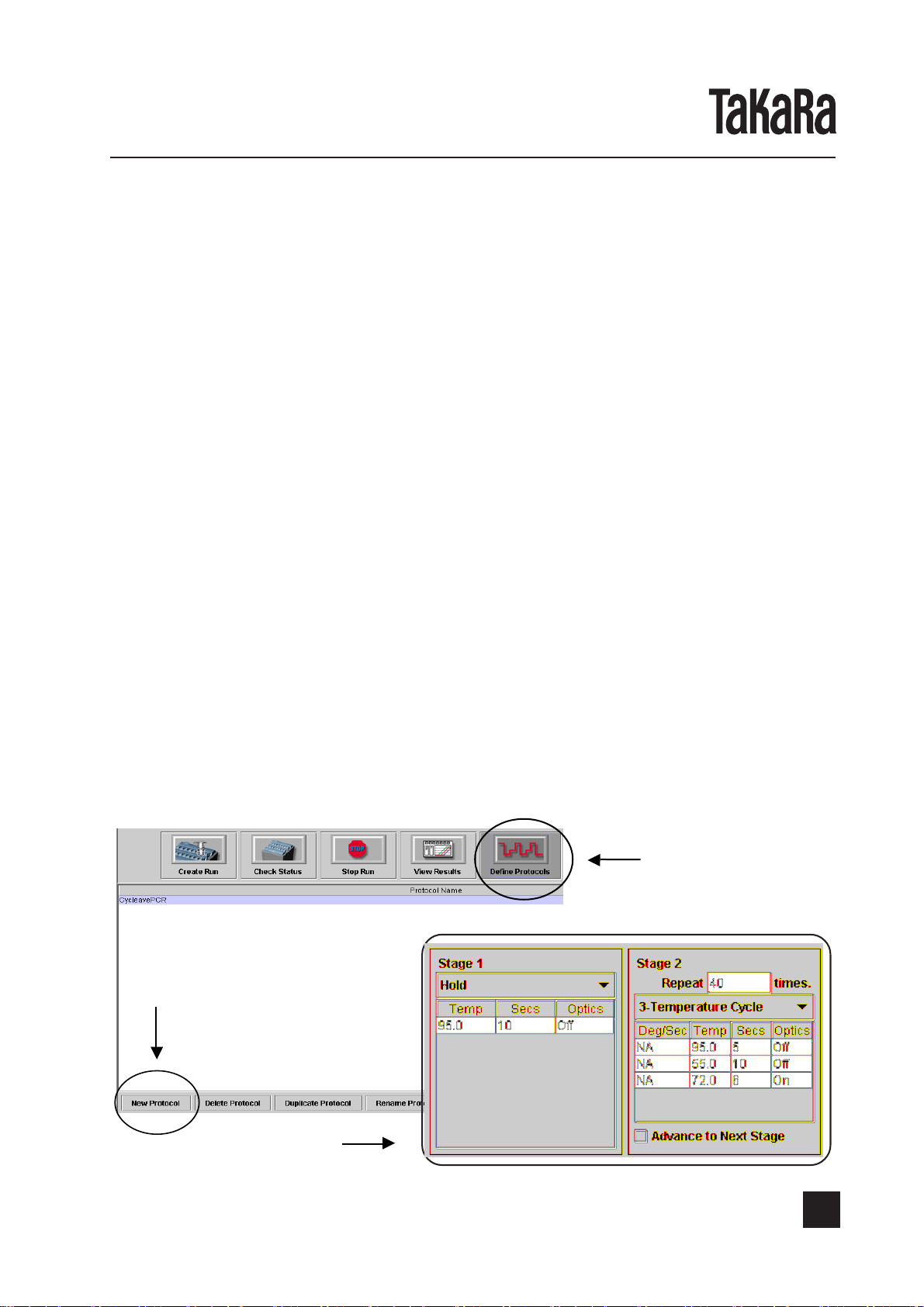
This Kit is for real-time PCR assay to detect, in combination with a thermal cycler for real time
PCR, Smart Cycler®II System*1(Cepheid), verotoxin genes (VT1 and VT2 genes) which cause
EHEC-related food poisoning. PCR is a technique for amplifying specifically the fragments of
the genes of interest in a short period of time using a trace amount of DNA as template. The
cycle comprising three steps of denaturation, primer annealing and extension with DNA
polymerase are repeated, thereby amplifying the gene fragments of interest up to 106-fold in
quite a short period of time.
By utilizing Smart Cycler®System, the amplification process can be real-time monitored.
As this kit uses Takara’s PCR enzyme efficient for Hot Start PCR,
Takara Ex TaqTM
R-PCR,
non-specific amplification deriving from mispriming or from primer-dimers before thermal
cycling can be avoided and it achieves highly sensitive detection.
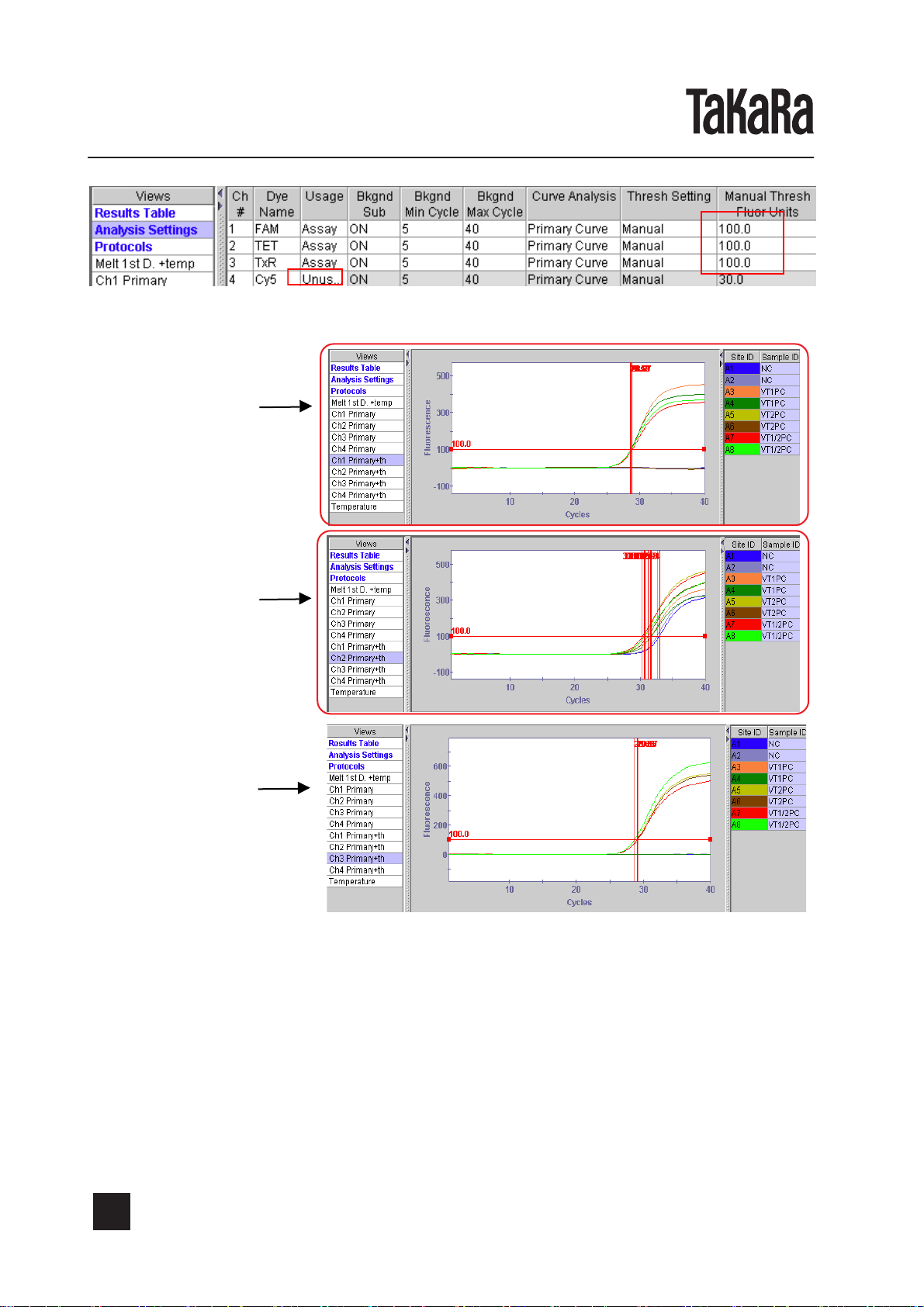
This kit employs Cycling Probe Technology(CPT)*2for detection, which is a high-sensitive
detection method utilizing a combination of chimera probe, composed of RNA and DNA, and
RNase H. The specific sequence of target gene to be amplified can be detected efficiently
during or after amplification by this method. The 5' end of the probe is labeled with a fluores-
cent substance and the 3' end is labeled with a quencher, which quenches the fluorescence
emitted from the fluorescent substance at the 5' end. As long as this probe remains intact, no
strong fluorescence can be emitted because of the quenching function. When this probe forms
a hybrid with the complementary sequence of amplified product, RNase H specifically cuts the
RNA region of this probe, resulting in emission of strong fluorescence. By measuring the
intensity of emitted fluorescence, the amount of amplified product can be monitored.
Version 2.0 achieves higher specificity by improving probes.
This Kit incorporates the FAM labeling probe for detecting VT1 and the ROX labeling probe for
detecting VT2. It also contains an internal control and the TET labeling probe for detecting the
internal control. Simultaneous monitoring of three wavelengths with Smart Cycler®II System
requires only one tube to distinguish VT1 from VT2. This Kit also is able to monitor a false
negative reaction through the use of internal control. The real-time detection with this Kit does
not require electrophoresis and the detection result can be obtained quickly (in about 30
minutes).
*1: Smart Cycler®System is a registered trademark of Cepheid.
*2: Cycling Probe Technology and DNA-RNA-DNA chimeric nucleic acid technology are
licensed by ID Biomedical Corporation.