TELEMED ArtUs EXT-1H User manual

TELEMED ArtUs User Guide, REV 1.6 2022.04.20
1
ArtUs EXT-1H
ArtUs EXT-2H
Ultrasound Diagnostic System
USER GUIDE

TELEMED ArtUs User Guide, REV 1.6 2022.04.20
2
Manufactured by
TELEMED UAB
Savanoriu pr. 178A
Vilnius, LT-03154
Lithuania
Telephones: (+370-5) 2106272 (+370-5) 2106273
Fax: (+370-5) 2306733
Internet: http://www.telemed.lt
E-Mail: [email protected]
NOTE: Non-TELEMED product names may be trademarks or registered trademarks
of their respective owners.
TELEMED ArtUs User Guide, REV 1.6 2022.04.20
3
1.INTRODUCTION............................................................................................ 5
1.1. ...About the system / Intended use ....................................................................5
1.2. ...Delivery set .....................................................................................................6
1.3. ...About the system software..............................................................................7
1.4. ...Technical Specifications .................................................................................8
2.SAFETY ....................................................................................................... 12
2.1. ...Electrical safety.............................................................................................12
2.2. ...Equipment protection ....................................................................................13
2.3. ...Biological safety............................................................................................14
2.4. ...Ultrasound exposure and ALARA principle...................................................14
2.5. ...Cybersecurity................................................................................................15
2.5.1. Information Security ................................................................................................ 15
2.5.2. Network Security ..................................................................................................... 15
2.5.3. Confidentiality.......................................................................................................... 16
2.5.4. Integrity.................................................................................................................... 16
2.5.5. Accountability .......................................................................................................... 16
2.6. ...Measurement Accuracy ................................................................................16
3.LABELING................................................................................................... 19
4.SYSTEM OVERVIEW.................................................................................. 20
4.1. ...Principle of operation ....................................................................................20
4.2. ...Components & Modifications ........................................................................21
4.2.1. Basic unit / Beamformer.......................................................................................... 21
4.2.2. Transducer Unit....................................................................................................... 21
4.3. ...Peripherals/Compatibility ..............................................................................22
5.USING THE SYSTEM.................................................................................. 23
5.1. ...INSTALLATION WARNINGS........................................................................23
5.2. ...Getting Started..............................................................................................24
5.3. ...Ultrasound Scanner Monitor utility ................................................................25
5.4. ...Windows Configuring ....................................................................................28
5.4.1. E-mail ...................................................................................................................... 28
5.4.2. Windows account .................................................................................................... 28
5.4.3.Windows security .................................................................................................... 28
5.4.4. Antivirus .................................................................................................................. 28
5.4.5. Firewall .................................................................................................................... 29
5.4.6. Windows updates.................................................................................................... 29
5.4.7. Network communication.......................................................................................... 29
5.4.8. Digital Signature ...................................................................................................... 29
5.4.9. Windows AppLocker ............................................................................................... 30
5.4.10. Encrypted file system .............................................................................................. 30
5.5. ...Finishing Work ..............................................................................................30
6.TROUBLESHOOTING ................................................................................ 31
6.1. ...FAQ ..............................................................................................................31
6.2. ...Contact with technical support service..........................................................31
7.WARRANTY AND SERVICE INFORMATION............................................ 33
7.1. ...Warranty .......................................................................................................33
7.2. ...Warranty Shipments and Returns.................................................................33
TELEMED ArtUs User Guide, REV 1.6 2022.04.20
4
7.3. ...Service Contract ...........................................................................................33
8.MAINTENANCE........................................................................................... 34
8.1. ...General cleaning...........................................................................................34
8.2. ...Inspecting the System...................................................................................34
8.3. ...Transducers maintenance and disinfection...................................................34
8.3.1. Chemicals that Damage Transducers:.................................................................... 35
8.3.2. Recommended Procedures for Transducer Processing ......................................... 36
8.3.3. General Cleansing for Transducers Used in Non-Invasive Procedures ................. 36
8.3.4. Cleansing and Disinfection of Transducers Used in Endocavity Procedures ......... 37
8.4. ...System Accuracy / Performance Verification ................................................37
9.TRANSPORTATION, STORAGE AND UTILIZATION ............................... 38
9.1. ...Transportation and storage...........................................................................38
9.2. ...Disposal ........................................................................................................38
10. ........DECLARATION OF CONFORMITY................................................... 39
11. ........APPENDICES ..................................................................................... 40
11.1..Guidelines for the safe use of diagnostic ultrasound.....................................40
11.2..Acoustic Output.............................................................................................49
11.3..Vigilance system ...........................................................................................49
11.4..Returned product form ..................................................................................51
12. ........REVISION HISTORY .......................................................................... 53

TELEMED ArtUs User Guide, REV 1.6 2022.04.20
5
1. INTRODUCTION
CAUTION:
United States federal law restricts this device to be used by, or on the
order of, a licensed physician.
Dear customer,
ArtUs EXT-1H/2H system is intended for multipurpose ultrasound
examinations, based on electronic linear and convex scanning.
It is an ideal budget solution for hospitals, specialized diagnostic centers,
public and private clinics.
Here in the User Guide you can find information about ArtUs EXT-1H/2H and
its safety and maintenance information.
Echo Wave II Software Operation Manual contains a description of the
controls.
1.1. About the system / Intended use
ArtUs EXT-1H/2H system is intended to be used for applications in fetal,
abdominal, pediatric, small organ (breast, thyroid, testicles), adult cephalic, musculo-
skeletal (conventional), musculo-skeletal (superficial) cardiac adult, cardiac pediatric,
peripheral vessel (B and M-mode, combined modes imaging, including imaging for
needle guidance) It is possible to provide diagnostic information outside of an
imaging lab, including at the bedside systems, for navigated medical applications and
in operating rooms/critical care units.
ArtUs EXT-1H/2H ultrasound systems provide many different scanning
technologies: B, B+B, 4B, B+M, M, CFM, Tissue Harmonic Imaging (THI). Echo
images can be either full size or zoomed.
Unlike ordinary ultrasound devices, this scanner is based on modern digital
technologies. PC application enables many powerful innovative features such as:
•user friendly, easy-to-use intuitive graphic user interface
•echo image storage on hard disk or other devices
•storage of a sequence of full-size echo images (cine) with the possibility to save
it in video file format
•image and cine file formats enable using other applications for viewing stored
data
•using a variety of peripheral devices
•image and video sending by E-mail.
A variety of available ultrasound transducers provides many different
applications for examinations in therapy, obstetrics, gynecology, urology, pediatrics,
oncology and other areas.

TELEMED ArtUs User Guide, REV 1.6 2022.04.20
6
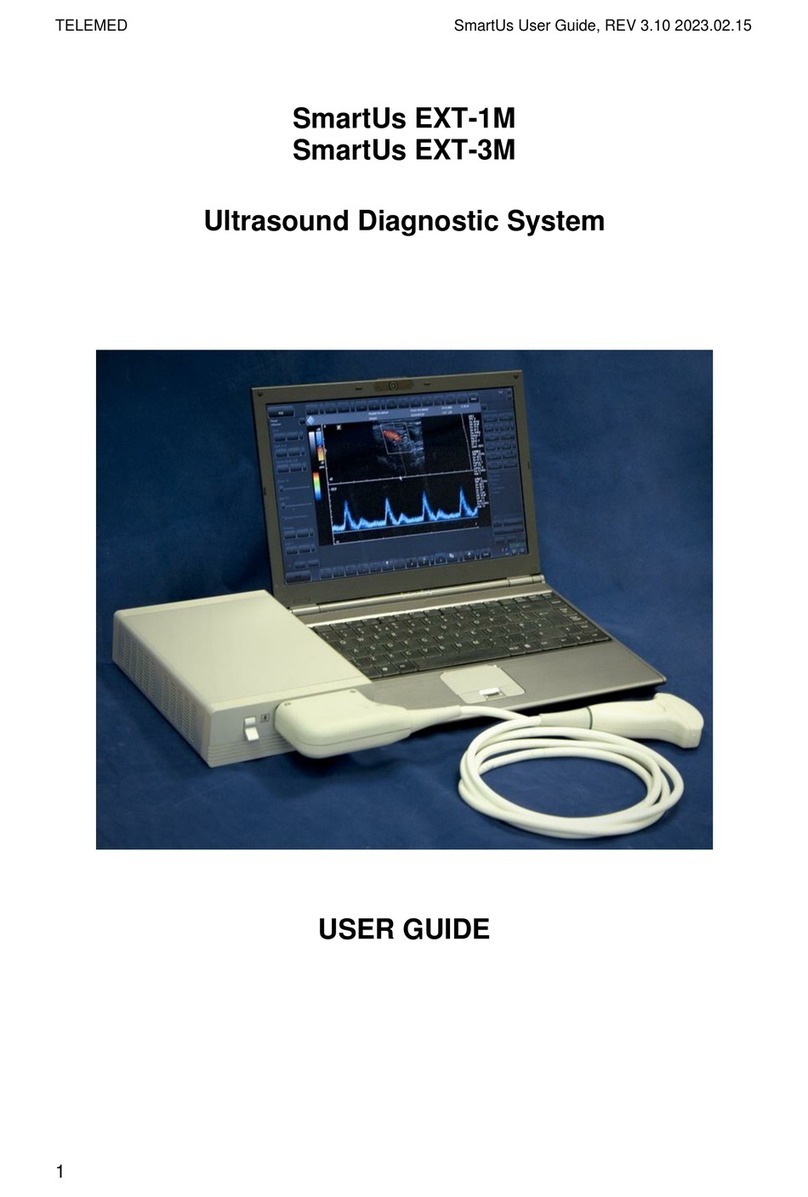
Common view of ArtUs EXT-1H is shown below (without transducer).
Common view of ArtUs EXT-1H is shown below (without transducer).
1.2. Delivery set
Beamformer
●
Operation Manual
●
This User Guide
●
Software and manuals (eIFU)
●
USB cable
●
Power supply (medical grade)
●
Ultrasound transducer(s)
Types and quantity
defined by customer

TELEMED ArtUs User Guide, REV 1.6 2022.04.20
7
1.3. About the system software
Your diagnostic system contains Echo Wave II software to control its
operation. TELEMED provides the latest Echo Wave II software version and drivers
package together with your system. In the software the unique technologies making
the intellectual property of TELEMED company are used. Latest software versions
can be downloaded directly on the Internet from http://www.telemed.lt.

TELEMED ArtUs User Guide, REV 1.6 2022.04.20
8
1.4. Technical Specifications
Table 1 contains technical specifications of ArtUs EXT-1H/2H.
Table 1
IMAGING MODES
1. B
2. B+B
3. 4B
4. B+M
5. M
6. B-steer for linear transducers
7. Compound for linear and convex transducers
8. Virtual convex for linear transducers
9. Expanded view angle for convex transducers
10. Color Doppler (CFM)
11. Power Doppler (PDI)
12. Directional Power Doppler (DPDI)
13. Pulsed Wave Doppler (PWD)
14. B+PWD (Duplex)
15. Inverted Tissue Harmonic Imaging (ITHI)
16. Tissue Harmonic Imaging (THI)
17. Parallel beam forming
18. RF data access using SDK library
ULTRASOUND IMAGING
1. ultrasound image size: automatically adjustable to screen resolution
2. gray scale: 256
3. color scale: 256
4. full motion and full-size real-time ultrasound imaging, up to 120 fps (depends on
selected scanning depth, angle, focusing mode, Lines Density setting, computer
speed)
5. cine recording/play: several thousand frames (depends on computer memory
size and scanning mode)
6. zoom mode: from 60% to 600% in all modes (Scan, Freeze, B, B+B, 4B, Doppler
modes, M-zoom, cine, etc.)
7. variable view area for maximizing frame rate: 6 steps
8. "FREEZE" mode
SCANNING METHOD
1. Electronic linear
2. Electronic convex
3. Electronic micro-convex
COLOR DOPPLER
1. PRF variable: 0.5-10 kHz
2. Wall filter settings: 3 steps (5%, %10%, 15% PRF)
3. Gain control: 40 dB
4. Angle steering for linear transducers: ±25°
TELEMED ArtUs User Guide, REV 1.6 2022.04.20
9
5. Real-time spatial filter: 4 values
6. CFM palette: 10 maps
7. B/Color priority control
8. Color threshold control
9. CFM baseline control
10. Doppler frequency selection: 2-3 frequencies for each transducer
11. Color frame averaging: 8 values
DEPTH SELECTION
1. 2 –30 cm (depth range depends on transducer type)
TRANSDUCERS
1. Ranging from 1.5 MHz to 18 MHz
2. Multi-frequency
3. Automatic transducer recognition
FOCUSING
4. Transmit: variable, 8 zones
5. Receive: point to point, dynamic
SIGNAL PROCESSING
1. Lines density control for better resolution
2. TGC control
3. Dynamic range
4. Overall gain control
5. M - mode sweep speed control
6. Acoustic power control
7. Variable frame averaging
8. Brightness, contrast
9. Advanced gamma control: 8 fixed curves, 8 user defined (custom)
10. Scan direction, rotation, up-down controls
11. Negative / positive control
12. Bi-linear interpolation
13. Echo enhancement control
14. Noise rejection function
15. Speckle reduction function
FUNCTIONS
General Measurements
and Calculations
•Mouse / trackball / keyboard operation of multiple calipers
•B-mode: Distance / Length / Area / Circumference / Volume
/ Angle / Stenosis % / A/B Ratio
•M-mode: Distance / Time / Velocity / Heart Rate / Stenosis
% / A/B Ratio
TELEMED ArtUs User Guide, REV 1.6 2022.04.20
10
Human Measurements
and Calculation
Packages
•General calculations package
•Obstetrics / Gynecology (OB / GYN) calculations package
•Gynecology (GYN)
•Abdominal exam measurements and calculations
•Urology
•Endocrinology
•Vascular exam measurements and calculations
•Cardiology
User Interface
•The set of predefined skin schemes for user interface
•User-friendly pop-up menus and dialog boxes
•Unlimited programmable presets for clinically specific
imaging
•Image comment / save / recall browsing
•Anatomical icons with transducer position indicator
Image and video save /
load
•JPG BMP PNG TIF AVI DCM DCM-JPG TVD TPD
Cine
•Recording up to 2048 frames to memory
•Play / Pause / Stop / Frame selection
•Saving ultrasound video file to disk
•Loading ultrasound video file from disk
Printing
•System printer
Internet
•Direct E-mail sending function with image or video
attachment
TV output
•Standard TV output using computer's display adapter
(option)
ULTRASOUND SOFTWARE
Drivers
•TELEMED Drivers Package
Software
•Echo Wave II software (B/W + Doppler modes)
DIMENSIONS AND WEIGHT
Dimensions W x D x H,
mm
136 x 189 x 28 (ArtUs EXT-1H)
140 x 205 x 65 (ArtUs EXT-2H)
Weight, kg
0.66 (ArtUs EXT-1H)
1.10 (ArtUs EXT-2H)
POWER CONSUMPTION
12 VDC, 3.5 A Max
•External AC medical grade power supply (100-240 VAC, 50-
60 Hz), Class II, XP Power AKM65US12C2
TELEMED ArtUs User Guide, REV 1.6 2022.04.20
11
5 VDC, 0.13 A Max
•USB 3.0 connection
SAFETY
Electromechanical safety
•IEC 60601-1 Medical electrical equipment part 1: General
requirements for safety.
Class II Type BF applied part
EMC/EMI standards
•European Norm EN 55011:1998 (CISPR 11:1999)
Industrial, scientific and medical (ISM) radio-frequency
equipment. Radio disturbance characteristics. Limits and
methods of measurement
Ultrasound exposure
•CEI/IEC 61157:1992, International Electrotechnical
Commission, Requirements for The Declaration of the
Acoustic Output of Medical Diagnostic Ultrasonic Equipment
•AIUM/NEMA: Standard for real-time display of thermal and
mechanical acoustic output indices on diagnostic ultrasound
equipment.1992
Degree of protection
(watertight)
•Main unit IPX0
•Transducers IPX7 (only the area of the transducer array
acoustic window)
OPERATIONAL ENVIRONMENT
Nominal operational
environment
•Environment temperature: 10 - 40 ° C
•Relative humidity not to exceed: 85 %
•Atmospheric pressure: 70 - 106 kPa

TELEMED ArtUs User Guide, REV 1.6 2022.04.20
12
2. SAFETY
CAUTION:
Please read this information before using the diagnostic system. It
applies to the ultrasound system, transducers, accessories and
peripherals.
2.1. Electrical safety
This system complies with the applicable medical equipment requirements and
meets IEC 60601-1, Class I Type BF safety requirements.
NOTE:
All persons connecting computer equipment as medical appliance
are configuring a medical system and are therefore responsible for
ensuring that the system complies with IEC 60601-1. The
achievement of PC compliance with the IEC 60601-1 requirements is
based on electrical safety. A standard PC power supply is almost
certain to not comply with IEC 60601-1 electrical requirements in
several ways, e.g. leakage current requirements, dielectric strength
requirements.
One possible solution is powering the PC (and computer monitor)
via a 1:1 medical insulation transformer, which has been designed to
meet IEC 60601-1 requirements. The best solution is a fully IEC
60601-1 certified PC or a battery-operated portable PC and wireless
peripheral devices.
All systems (including monitors and other connected parts) must be
configured to comply with IEC 60601-1. If in any doubt please
contact the technical service department of your local
representative.
Note that regardless of the above stipulations all personal
computers used should be approved regarding the IT (information
technology) safety standards for electrical equipment (such as IEC
60950 or equivalent).
The electrical specification is shown below and is labeled on the rear panel of
a scanner.
To avoid electrical shock only use the supplied cables and connect it to
properly earthed power socket. Do not use a three pin - two pin adapter. This defeats
the whole purpose of earthing for safety reasons. Systems should be operated within
the voltage limits.
If the ultrasound scanner will be moved or left unused for a long period of time
without being switched on it is recommended to disconnect it from a power supply.
WARNING:
In the event of detecting a discrepancy regarding patient safety
requirements (occurrence or probability of risk) you must to inform
the local dealer and the manufacturer immediately.
TELEMED ArtUs User Guide, REV 1.6 2022.04.20
13
If a scanner is to be switched on, do not interrupt this while operating the
system and while the ultrasound software is being loaded. The time for this operation
is approximately 1 minute.
To avoid the risk of electrical shock and fire hazard:
•before using the transducer, inspect the transducer face, housing, and
cable and do not use the transducer if the transducer or the cable is
damaged;
•always disconnect the AC power supply from the system before cleaning
the system;
•do not use any transducer that has been immersed beyond the specified
cleaning or disinfection level;
•inspect the power supply, AC power supply cable and electrical plug on a
regular basis to ensure they are not damaged;
•do not supply power to the system from unapproved AC power supply, not
supplied by TELEMED;
•only use accessories and peripherals recommended by TELEMED.
WARNING:
To allow quick and easy disconnecting of equipment from power
source in case of emergency place the equipment so that there is a
gap at least 10 cm between the rear panel and the other object (wall,
toher equipment, tec.) and the power plug is accessible by hand.
WARNING:
To avoid the risk of electrical shock do not open the cover of device.
There are no parts that you can repair yourself. In case of difficulties
please contact the TELEMED service department or your nearest
local authorized distributor.
2.2. Equipment protection
To protect your ultrasound system, transducer and accessories, please follow
these precautions:
•excessive bending or twisting of electrical cables can cause a failure or
intermittent operation;
•incorrect cleaning or disinfecting of any system part can cause
permanent damage, for cleaning and disinfecting instructions see the
relevant chapter below;
•do not use solvents such as thinners/benzene or abrasive cleaners on
any parts of the system;
•do not spill liquids on the system;
•incorrect assembly or configuration and using an incorrect power source
may damage the system.
WARNING:
Ultrasound transducers can easily be damaged by incorrect
handling! Failure to follow these precautions can result in serious
injury and equipment damage!
TELEMED ArtUs User Guide, REV 1.6 2022.04.20
14
2.3. Biological safety
Observe the following precautions related to biological safety:
•do not use the system if it displays erratic or inconsistent behavior;
•interruptions to the scanning sequence are signs of hardware failure that
must be corrected before use;
•do not use the system if it displays artifacts on the LCD screen, either
within the clinical image or on the area outside it;
•artifacts are indications of hardware and/or software errors that must be
corrected before use;
•perform ultrasound procedures prudently, use the ALARA (As low As
Reasonably Achievable) principle (see APPENDIX: Guidelines for the safe
use of diagnostic ultrasound);
•devices are contraindicated for ophthalmic use or any application that causes
the acoustic beam to pass through the eye.
WARNING:
At detection of discrepancy to patient’s safety requirements (occurrence or
probability of risk) you need to inform immediately the local dealer and the
manufacturer.
2.4. Ultrasound exposure and ALARA principle
Perform ultrasound procedures prudently, use the ALARA (As low As
Reasonably Achievable) principle (see APPENDIX: Guidelines for the safe use of
diagnostic ultrasound).
The interactive system features or user controls that may affect the acoustic
output are:
•acoustic output control,
•transmit frequency;
•scanning depth;
•transmit focal length;
•scanning angle.
Acoustic output also depends on the imaging mode selected. The choice of
mode (B-Mode, M-Mode, B+M-Mode) determines whether the ultrasound beam is
stationary or in motion. B+M-Mode has the highest acoustic output.
The default output level is factory calibrated and is based on device settings
that yield an optimum image for the type of patient examination and do not exceed
the following FDA recommended limits.
WARNING: Some transducer covers may contain talc and natural rubber
latex. Examine the package labeling to confirm latex content. We
strongly recommend that health-care professionals identify their latex-
sensitive patients, and refer to the FDA’s March 29, 1991 Medical Alert on
Latex products. Be prepared to treat allergic reactions promptly.
NOTE: TELEMED diagnostic ultrasound systems and transducers do not
contain natural rubber latex that contacts humans.
TELEMED ArtUs User Guide, REV 1.6 2022.04.20
15
This default level is set:
•when the system is first turned on;
•when the transducer is first turned on.
It is highly recommended to set the default level:
•when changing from one exam category to another;
•when changing from one application to another;
•when changing from one transducer to another;
•when a new patient is entered.
Once an optimal image is achieved, the need for increasing acoustic output or
prolonging the exposure cannot be justified. Watch the POWER level (on-screen
display) permanently. Whenever possible, controls and system features should be
used to optimize the image before increasing the acoustic output level. Follow the
ALARA principle during all patient examinations.
The ArtUs devices employ the ALARA principle in configuring factory defaults.
Ultrasound waves used in diagnostic system have frequencies ranging from 2
MHz to 18 MHz Sound waves with such frequencies are weakened in the air, so can
be measured, for example, in water. Ultrasound waves sent by a converter are so
weak (medium intensity less than 100 mW/cm²), that, according to International
Electrotechnical Commission (IEC 1157) standards (well within AIUM/NEMA
standards), they do not have any impact on patient health (however any unnecessary
exposure should be avoided).
Detailed information is found in
APPENDIX: Guidelines for the safe use of diagnostic ultrasound.
2.5. Cybersecurity
Vulnerabilities in cybersecurity may represent a risk to the safe and effective
operation of networked medical devices. Store only relevant and necessary software
on working computers.
Network administrators in healthcare organizations and information technology
providers should assure an adequate degree of protection from threats such as
viruses and worms to avoid the risk of any unauthorized access to the network or the
medical device/database. Please share with your local administrator detailed settings
information from this document section “Windows configuring”.
2.5.1. Information Security
When entering and saving data it is your responsibility to protect your security
credentials and the personal information of patients.
2.5.2. Network Security
CONTRAINDICATION:
This device is contraindicated for ophthalmic use or any application that
causes the acoustic beam to pass through the eye.

TELEMED ArtUs User Guide, REV 1.6 2022.04.20
16
Use a network supporting Wi-Fi 802.11n and WPA (Wi-Fi Protected Access) or
WPA2 (Wi-Fi Protected Access II) as your security protocol.
Refer to your network equipment documentation for setting wireless network
security.
Do not use untrusted wireless access points, it may allow third party to perform
harmful actions. When no secure access point is available, operate in Wi-Fi Direct
mode –it will automatically set up encryption.
For security purposes:
•Use secure passwords
•Use secure protocols, secure wireless equipment with the latest
firmware/software
•Lock your PC
The following actions could introduce new risks to patients, operators and third
parties:
•Changing network configuration
•Connecting to additional networks or disconnecting from existing
networks
•Upgrading to new equipment or updating existing equipment
2.5.3. Confidentiality
If you want the data encrypted, connect to a:
•Wi-Fi network where only trusted parties are permitted. The Wi-Fi
network encrypts all image data sent from other Wi-Fi networks.
•Wi-Fi Direct network. The Wi-Fi Direct network encrypts all image data,
and because no other users are on the Wi-Fi Direct network, the image
data is confidential. Because Wi-Fi Direct network is a peer-to-peer
connection using the Wi-Fi protocol, it disallows other users from
connecting, thereby reducing DDOS (Distributed Denial of Service)
attacks.
2.5.4. Integrity
Integrity of the data transmitted between the device and network is
assured as follows:
•Authenticated encryption prevents malicious users from intercepting
and modifying data.
•TCP channels used over Wi-Fi ensures that data is delivered correctly.
2.5.5. Accountability
Ownership (i.e. the active user) of a PC is assigned to one user at a
time. Once you begin using the PC, no other user can connect to the same
device. All data transmitted between the device and network is owned by the
active user.
2.6. Measurement Accuracy

TELEMED ArtUs User Guide, REV 1.6 2022.04.20
17
The accuracy of measurements is determined not only by the TELEMED Echo
Wave II software but also by the proper use of medical protocols.
Distance and area/circumference measurements are displayed to 0.1 mm.
The following general assumptions can be made about the accuracy of any
ultrasound system:
•Velocity of sound is constant - 1540 m/s
•Velocity of sound uncertainty is 5%
•Caliper placement accuracy is one pixel (operator dependent)
•Measurement accuracy is based on the root-mean-square combination of
all independent sources of error
•RMS errors are due to velocity of sound uncertainty, pixel error, and
typical transducer geometry
Note: The below measurement accuracies apply to all transducers and to all modes.
The linear distance measurement components have the accuracy and range
shown in the following tables:
2D Measurement Accuracy
2D Measure
Accuracy and
Range
System Tolerance
*****
Accuracy
By
Test
Method
Range
Axial Distance
< ±2% or 1mm
Acquisition
Phantom**
0.1-20 cm
Lateral Distance
< ±2% or 1mm
Acquisition
Phantom**
0.1-20 cm
Diagonal Distance
< ±2% or 1mm
Acquisition
Phantom**
0.1-20 cm
Area ***
Trace & Ellipse
< ±4% plus 1% of full
scale*
Acquisition
Phantom**
0.1-1000
cm²
Circumference
****
< ±3% plus 1% of full
scale*
Acquisition
Phantom**
0.1-70 cm
Angle
< ±5%
Acquisition
Phantom**
0 -180º
* Full scale for distance implies the maximum depth of the image.
** An ATS model 539 phantom with 0.7 dB/cm-MHz attenuation was used.
*** The area accuracy is defined using the following equation: % tolerance = ((1 +
lateral error) * (1 + axial error) –1) * 100 + 0.5%.
**** The circumference accuracy is defined as the greater of the lateral or axial
accuracy and by the following equation: % tolerance = ((maximum of 2 errors) * 100)
+ 0.5%.
***** To take into account which of the tolerances is greater.
M-mode Measurement and Calculation Accuracy
WARNING:
Clinical diagnostic errors may result from the incorrect use of
calculations. Review the referenced source of the stated formula or
method to become familiar with the intended uses and possible
limitations of the calculations. Calculation formulas and databases
are provided as a tool to assist the user and should not be
considered as an undisputed database when making a clinical
diagnosis.

TELEMED ArtUs User Guide, REV 1.6 2022.04.20
18
M-mode
Measurement
Accuracy
and
Range
System Tolerance
Accuracy
By
Test
Method
Range
Distance
< ±5% or 1mm
Acquisition
Phantom **
0.1-20
cm
Time
< ±2% plus 1% of full scale *
Acquisition
Phantom****
0.1-10
sec
Heart Rate
< +/- 2%
+ (Full Scale *** x Heart
Rate/100) %
Acquisition
Phantom****
20-300
bpm
* Full scale for distance implies the maximum depth of the image.
** An ATS model 539 phantom with 0.7 dB/cm-MHz attenuation was used.
*** Full scale for time implies the total time displayed on the scrolling graphic image.
**** TELEMED special test equipment was used.
Other Measurement and Calculation Accuracy
Parameter
System
Tolerance
Reference / Formula
Volume
< ±9%
4.2.3 Perimeter, square and
volume measurements by
Ellipse method
Fetus Weight
1 method
< ±16%
4.5.1 Hadlock85 (USA)
2 method
< ±12%
4.5.2 Shepard82 (EU)
3 method
< ±17%
4.5.3 Tokyo
4 method
< ±16%
4.5.4 Osaka
Left Ventricle Volume
1 method
< ±15%
4.6.2 Cubed
2 method
< ±11%
4.6.2 Pombo
3 method
< ±13%
4.6.2 Teichholz
Stroke Volume
< ±15%
4.6.3 Stroke Volume
Ejection Fraction
< ±12%
4.6.4 Ejection Fraction
Cardiac Output
< ±15%
4.6.5 Cardiac Output
Left Ventricle Internal Dimension
Fractional Shortening
< ±10%
4.6.6 Left Ventricle Internal
Dimension Fractional
Shortening
Aortic Valve Measurements and
Calculations
< ±8%
4.6.7 Aortic Valve
Measurements and
Calculations
TELEMED ArtUs User Guide, REV 1.6 2022.04.20
19
3. LABELING
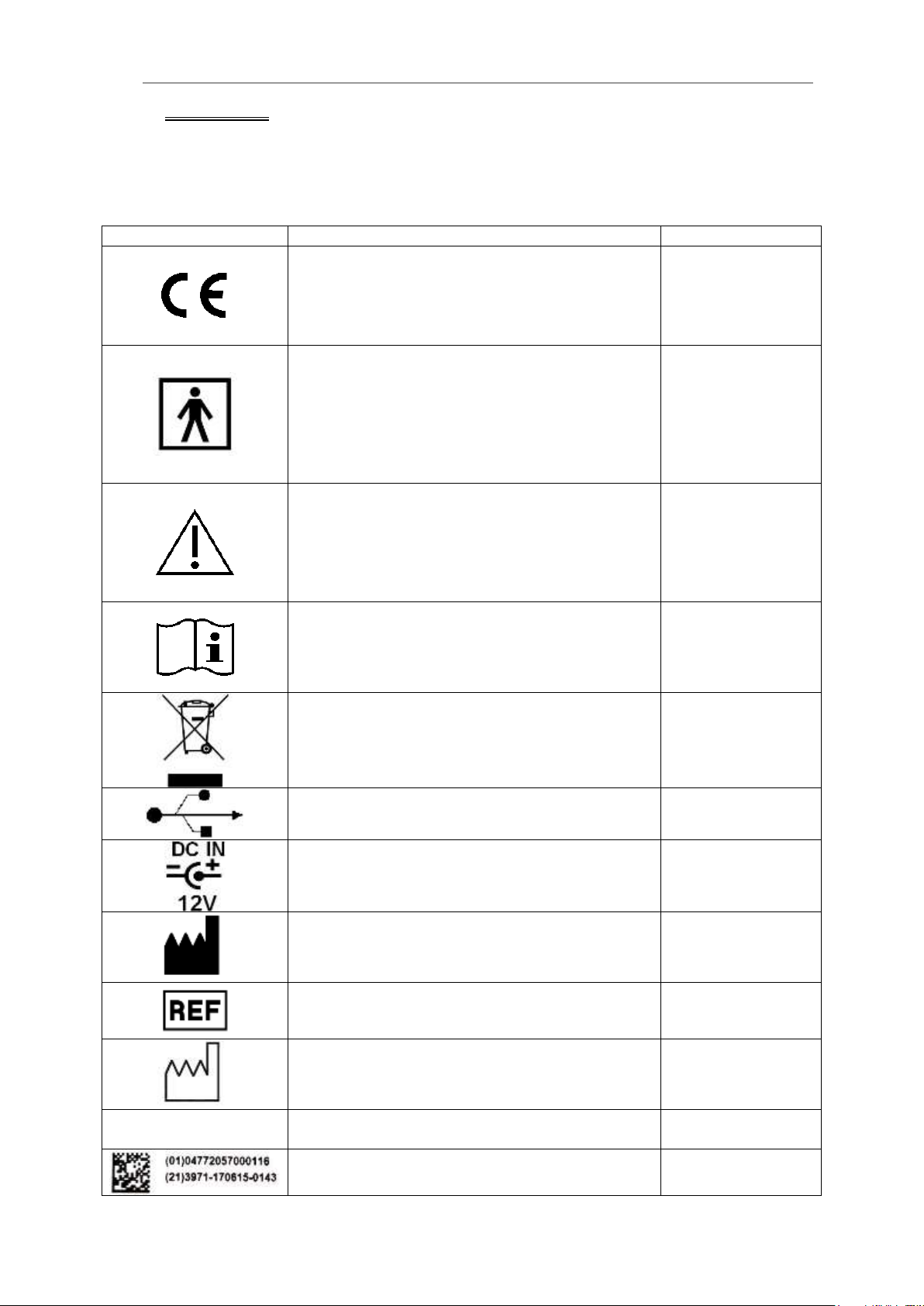
Table 2 describes the purpose and location of safety labels and other
important information provided on the equipment.
Table 2
LABEL/SYMBOL
DESCRIPTION
LOCATION
CE mark
This mark is a declaration by the manufacturer
that the respective component complies with
the relevant directives and standards as issued
by the European Union.
Rear panel (rating
plate label)
Type BF Equipment (man symbol) IEC 878-
02-03 indicates BF type equipment which
provides a particular degree of protection
against electric shocks, particularly regarding
allowable LEAKAGE CURRENT and reliability
of the PROTECTIVE EARTH CONNECTION if
present.
External
(transducer outlet)
Caution, consult accompanying documents
This symbol advises the reader to consult the
accompanying documents for important safety-
related information such as warnings and
precautions that cannot, for a variety of
reasons, be presented on the device itself
Rear panel (along
with rating plate
label)
Consult instructions for use
This symbol advises the reader to consult the
operating instructions for information needed
for the proper use of the device
Rear panel (along
with rating plate
label)
The symbol indicating separate collection for
electrical and electronic equipment (Annex IV
of Directive 2002/96/EC)
Rear/bottom panel
USB connector
Rear panel
DC power input
Rear panel
Manufacturer name and address
ID Label
Model / Catalogue number
ID Label
Date of manufacture
YEAR -MONTH- DAY
ID Label
IPX7
Protection (watertight, only the area of the
transducer acoustic window)
Transducer
UDI GS1 Data Matrix 2D barcode
ID Label
Transducer

TELEMED ArtUs User Guide, REV 1.6 2022.04.20
20
4. SYSTEM OVERVIEW
The ArtUs EXT-1H/2H system handles the multi-element transducers.
Here is main information about Ultrasound Scanner. The system consists of, see
figure below:
•Beamformer
•Power Supply +12VDC
•Ultrasound Transducer
•Windows PC (Desktop / Notebook / Tablet PC) with integrated USB 3.0 port
Attention:
ArtUs system requires Windows PC with integrated USB 3.0 or better port.
For more technical details please refer to 5.2 paragraph.
4.1. Principle of operation
The ultrasound diagnostic system is based on the effect of ultrasound wave
reflection from the tissue edges with different acoustic impedance levels. Ultrasound
waves sent out by the transducer head are emitted into the patient’s body.
Reflections from the specific types of tissue and their external surface/edges cause
partial reflections of the propagating sound wave. The return echo comes back to the
transducer head and after being detected and amplified is displayed on the monitor
screen as a pixel combination with various shades of brightness creating an
ultrasound image.
This manual suits for next models
1
Table of contents
Other TELEMED Diagnostic Equipment manuals