TELEMED MicrUs Pro User manual

Telemed 2020
Rev.1.9
MicrUs Pro Ultrasound System
User Guide

MicrUs Pro User Guide
- 2 -
Contents
Introduction.............................................................................................................4
1.1. About the System / Intended Use.....................................................................4
Safety and Regulatory Requirements.....................................................................5
2.1. Warnings..........................................................................................................5
2.2. Acoustic Output................................................................................................5
2.3. Electromagnetic Conformance (EMC)..............................................................6
Labeling................................................................................................................10
3.1. Symbols .........................................................................................................10
System Overview..................................................................................................11
Getting Started with Android System....................................................................11
5.1. System Requirements....................................................................................11
5.2. Software Installation.......................................................................................11
5.3. Connecting Android System...........................................................................12
5.4. Running Echo Wave A Application.................................................................12
Getting Started with Windows PC.........................................................................14
6.1. PC Hardware Requirements ..........................................................................14
6.2. Software Installation.......................................................................................15
6.3. Connecting MicrUs Pro to PC ........................................................................22
6.4. Running Echo Wave II Software ....................................................................22
6.5. Additional Recommendations for Cybersecurity.............................................24
Maintenance, Cleaning and Disinfecting...............................................................26
7.1. General Maintenance Rules...........................................................................26
7.2. Preparation for Cleaning Procedure...............................................................26
7.3. MicrUs Pro Cleaning ......................................................................................27
7.4. MicrUs Pro Low-Level Disinfecting.................................................................27
7.5. MicrUs Pro High-Level Disinfecting................................................................28
7.6. List of Compatible Cleaners and Disinfectants...............................................28
Specifications........................................................................................................29
8.1. Mobile Device Requirements .........................................................................29
8.2. System Specifications....................................................................................30
8.3. Essential Performance...................................................................................30
8.4. List of Parts and Accessories.........................................................................30
8.5. Operational and Storage Environment...........................................................31
Transportation, Storage and Disposal ..................................................................31
Declaration of Conformity .....................................................................................32

MicrUs Pro User Guide
- 3 -
Troubleshooting Q&A ...........................................................................................33
Warranty and Service Information ........................................................................33
12.1. Warranty.........................................................................................................33
12.2. Warranty Shipments and Returns..................................................................33
12.3. Obtaining Device Serial Number....................................................................34
12.4. Service Contract.............................................................................................35

MicrUs Pro User Guide
- 4 -
Introduction
Dear customer,
The MicrUs Pro ultrasound system is intended for the multipurpose ultrasound
examinations, based on electronic linear and convex scanning.
It is ideal budget solution for hospitals, specialized diagnostic centers, public / private
clinics.
Our new class of PC-based compact ultrasound systems is featuring:
•Scan-converter free architecture beamformer
•All-in-one solution: beamformer and transducer in one device
•Connectivity via fast USB Type-C interface to any Windows PC (Desktop,
Laptop or Tablet) or Android device
•USB powered and does not require external power supply
•Digitally controlled acoustic power
•Light weight, true mobility, fits the size of ordinary transducer.
Here in User Guide you can find a common information about the MicrUs Pro system,
how to assemble the components and install the software, safety and maintenance
information. Operation Manual contains Echo Wave II software description for Windows-
based device users and Echo Wave A for Android device users.
1.1. About the System / Intended Use
The MicrUs Pro ultrasound system is intended to be used for applications in cardiac (adult
and pediatric), fetal, abdominal, pediatric, small organ, peripheral vessel and musculo-
skeletal. The MicrUs Pro is a highly portable computer controlled ultrasound system used
to acquire and display real-time high-resolution ultrasound data in B-mode, M-mode, B+M-
mode (Windows) or B-mode (Android).
The systems have measurement capabilities for anatomical structures and fetal biometry
that provide clinical diagnostic information. It is possible to provide diagnostic information
outside of an imaging lab, including at the bedside systems, for navigated medical
application, in operating rooms/critical care units.
System offers to get a real-time and “frozen” echo images in all scanning modes. Unlike
ordinary ultrasound devices this scanner is based on modern digital technologies. PC and
USB application enables many powerful innovative features such as:
•user friendly, easy-to-use intuitive graphic user interface
•echo images storage on hard disk or any other storage devices
•storage of a sequence of full-size echo images (cineloop) with possibility to save it
in video file format
•telecommunication possibilities
•using a variety of peripheral devices.

MicrUs Pro User Guide
- 5 -
Safety and Regulatory Requirements
2.1. Warnings
Use of this equipment adjacent to other equipment should be avoided because it
could result in improper operation. If such use is necessary, this equipment and
the other equipment should be observed to verify that they are operating normally.
Use of accessories, transducers and cables other than those specified or provided
by the manufacturer of this equipment could result in increased electromagnetic
emissions or decreased electromagnetic immunity of this equipment and result in
improper operation.
Portable RF communications equipment (including peripherals such as antenna
cables and external antennas, but except the equipment which is a part of the
system) should be used no closer than 30 cm (12 inches) to any part of the
MicrUs Pro and cable attached. Otherwise, degradation of the performance of this
equipment could result.
2.2. Acoustic Output
Trained professionals should perform diagnostic ultrasound procedures safely for the
intended purpose. The MicrUs Pro and its thermal (TI) and mechanical (MI) safety limits
are set to industry standards, as a Track 3 device, and are not displayed on the display
screen, because they are not exceed the value of 1,0 at any given time, based on the all
possible setting configurations as per IEC 60601-2-37. Medical electrical equipment. Part
2-37: Particular requirements for the safety of ultrasonic medical diagnostic and monitoring
equipment.
MicrUs Pro User Guide
- 6 -
2.3. Electromagnetic Conformance (EMC)
The MicrUs Pro system is intended to enable diagnostic ultrasound imaging and
measurement of anatomical structures and fluids by qualified and trained healthcare
professionals. Electromagnetic fields, however, can cause distortion or degradation of this
information, affecting this performance. The MicrUs Pro system has been designed for
use within electromagnetic environments specified in tables below. To avoid radiated and
conducted electromagnetic disturbances, the customer or the user of the MicrUs Pro
system should assure that it is used within these stated specifications.
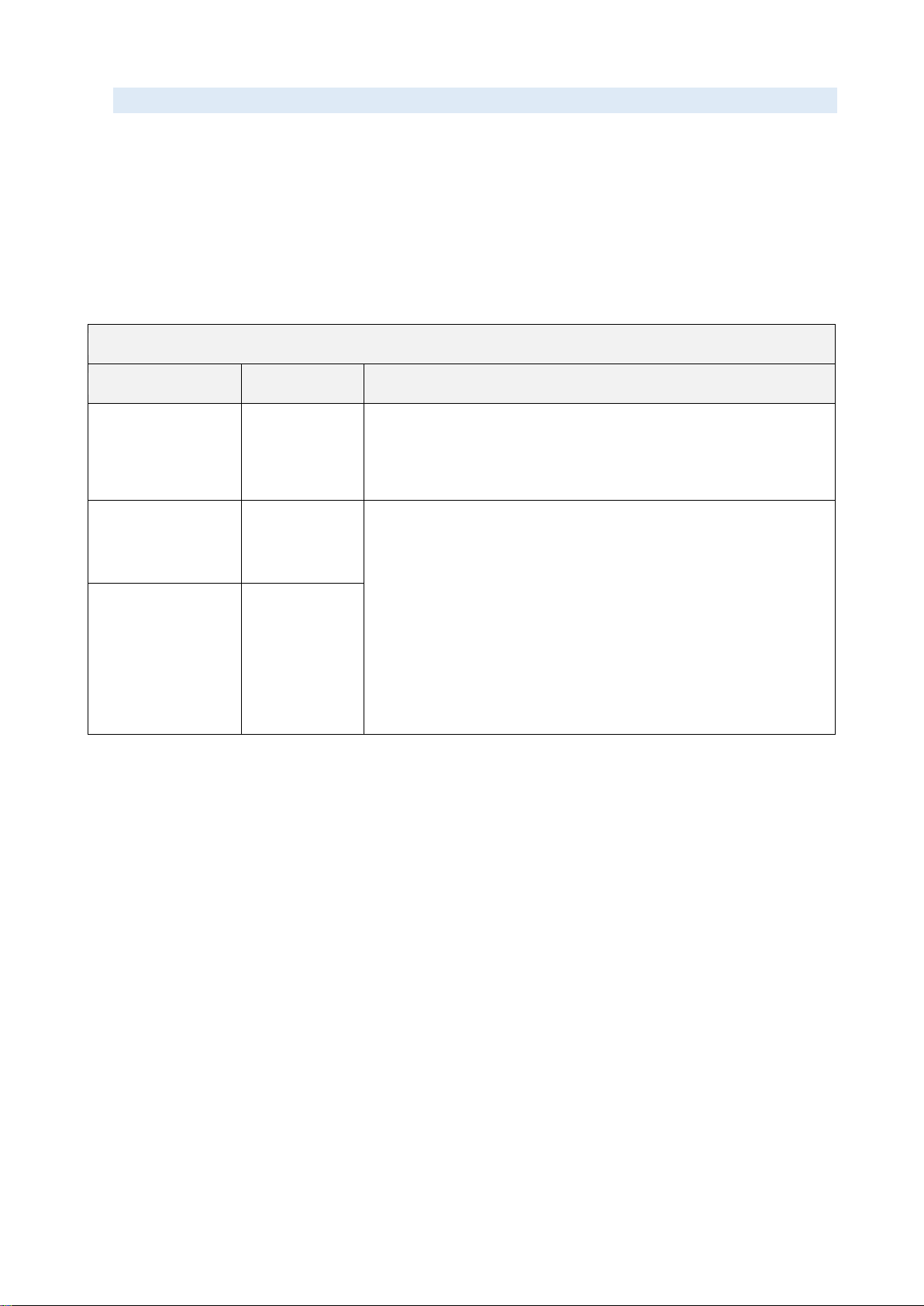
Declaration –electromagnetic emissions
Emissions test
Compliance
Electromagnetic environment –guidance
RF emissions
CISPR 11
Group 1
Class A
The MicrUs Pro uses RF energy only for its internal
function. Therefore, its RF emissions are very low and are
not likely to cause any interference in nearby electronic
equipment.
Harmonic
emissions
IEC 61000-3-2
Class A
The MicrUs Pro is suitable for use in all establishments
other than domestic, and may be used in domestic
establishments and those directly connected to the public
low-voltage power supply network that supplies buildings
used for domestic purposes, provided the following warning
is heeded: Warning: This equipment/system is intended for
use by healthcare professionals only. This equipment/
system may cause radio interference or may disrupt the
operation of nearby equipment. It may be necessary to take
mitigation measures, such as re-orienting or relocating the
MicrUs Pro system or shielding the location.
Voltage
Fluctuations and
Flicker
IEC 61000-3-
3:2013
Complies

MicrUs Pro User Guide
- 7 -
Declaration –electromagnetic immunity
IMMUNITY test
IEC 60601 test
level
Compliance
level
Electromagnetic environment
–
guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
8 kV contact
2, 4, 8, 15kV air
8 kV contact
2, 4, 8, 15kV
air
Floors should be wood,
concrete or ceramic tile. If
floors are covered with
synthetic material, the relative
humidity should be at least 30
%.
Electrical fast
transient/burst
IEC 61000-4-4
2 kV for power
supply lines
1 kV for
input/output lines
2 kV for
power supply
lines
N/A
Mains power quality should be
that of a typical commercial or
hospital environment.
Surge
IEC 61000-4-5
1 kV line(s) to
line(s)
2 kV line(s) to earth
2 kV Signal
input/output) to
earth
1 kV line(s) to
line(s)
2 kV line(s) to
earth
N/A
Mains power quality should be
that of a typical commercial or
hospital environment.
Voltage dips,
short
interruptions
and voltage
variations on
power supply
input lines
IEC 61000-4-11
0% UT; 0.5cycle at
0°, 45°, 90°,
135°,180°, 225°,
270° and 315°
0% UT; 1cycle and
70% UT; 25/30
cycles
Single phase at 0°
0% UT; 250/300
cycle
0% UT;
0.5cycle at
0°, 45°, 90°,
135°,180°,
225°, 270°
and 315°
0% UT;
1cycle and
70% UT;
25/30 cycles
Single phase
at 0° 0% UT;
250/300
cycle
Mains power quality should be
that of a typical commercial or
hospital environment. If the
user of the MicrUs Pro system
requires continued operation
during power mains
interruptions, it is
recommended that the MicrUs
Pro system be powered from
an uninterruptible power supply
or a battery.
Power
frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
30 (A/m)
30 (A/m)
Power frequency magnetic
fields should be at levels
characteristic of a typical
location in a typical commercial
or hospital environment.
NOTE UT is the AC mains voltage prior to application
of the test level.
MicrUs Pro User Guide
- 8 -
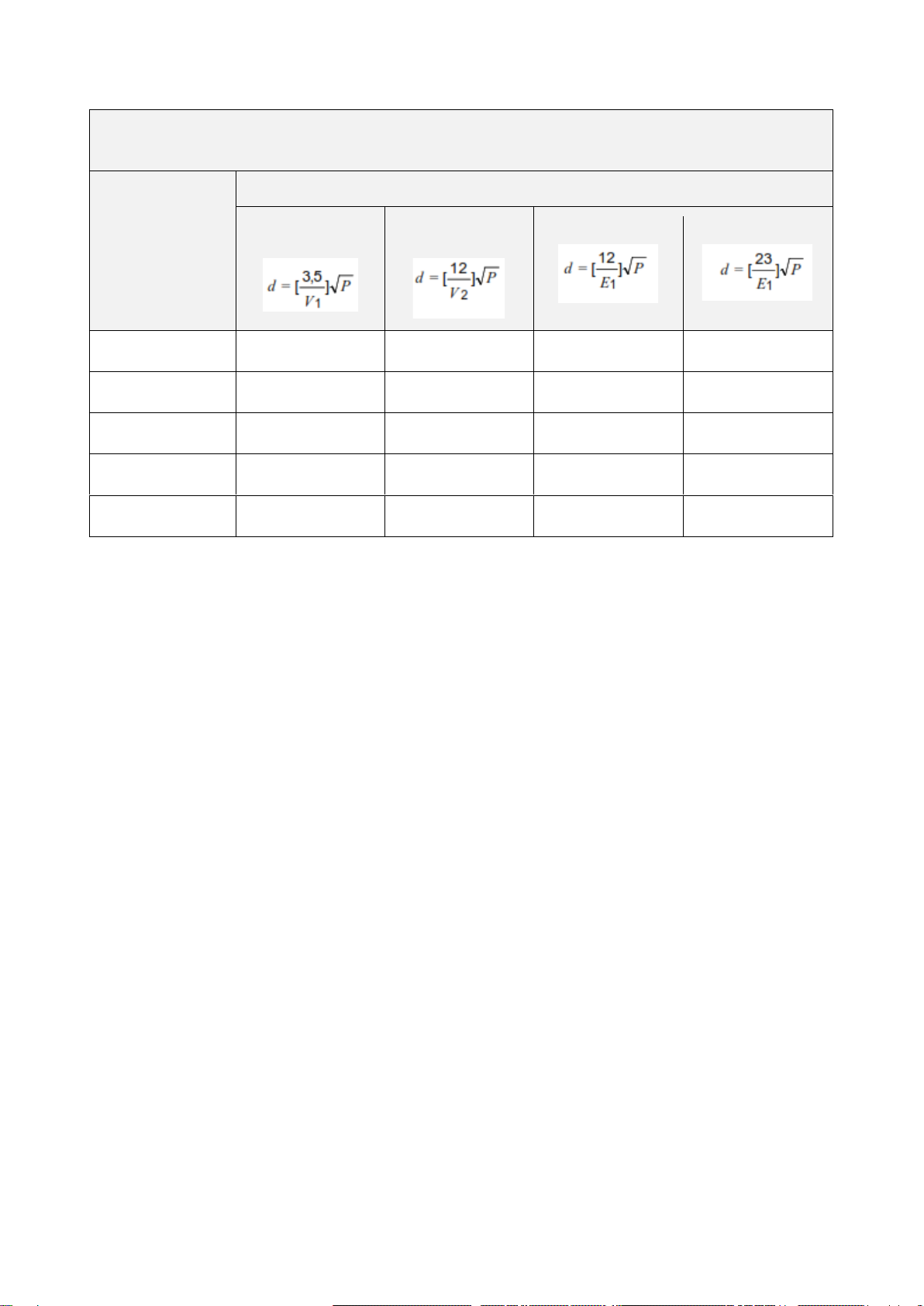
Recommended separation distances between portable and mobile RF
communications equipment and the MicrUs Pro system
Rated maximum
output power of
transmitter, W
Separation distance according to frequency of transmitter, m
150 kHz to 80 MHz
outside ISM bands
150 kHz to 80 MHz
in ISM bands
80 MHz to 800 MHz
800 MHz to 2,5 GHz
0.01
0.12
0.2
0.4
1
0.1
0.37
0.64
1.3
2.6
1
1.17
2
4
8
10
3.7
6.4
13
26
100
11.7
20
40
80

MicrUs Pro User Guide
- 9 -
Test specifications for ENCLOSURE PORT IMMUNITY to RF wireless
communications equipment
Test
frequency
(MHz)
Band
(MHz)
Service
Modulation
Max
power
(W)
Distance
(m)
IMMUNITY
TEST
LEVEL
(V/m)
Compliance
level
(V/m)
385
380 –
390
TETRA 400
Pulse
modulation
18 Hz
1.8
0.3
27
27
450
430 –
470
GMRS 460,
FRS 460
FM
± 5 kHz
deviation
1 kHz sine
2
0.3
28
28
710
704 –
787
LTE Band 13,
17
Pulse
modulation
217 Hz
0.2
0.3
9
9
745
780
810
800 –
960
GSM 800/900,
TETRA 800,
iDEN 820,
CDMA 850,
LTE Band 5
Pulse
modulation
18 Hz
2
0.3
28
28
870
930
1720
1 700
–
1 990
GSM 1800;
CDMA 1900;
GSM 1900;
DECT;
LTE Band 1,
3, 4, 25;
UMTS
Pulse
modulation
217 Hz
2
0.3
28
28
1845
1970
2450
2 400
–
2 570
Bluetooth,
WLAN,
802.11 b/g/n,
RFID 2450,
LTE Band 7
Pulse
modulation
217 Hz
2
0.3
28
28
5240
5 100
–
5 800
WLAN 802.11
a/n
Pulse
modulation
217 Hz
0.2
0.3
9
9
5500
5785
MicrUs Pro User Guide
- 10 -
Labeling
3.1. Symbols
You can find several symbols on your medical product and its packaging that classify a
connection, warn of potential hazards or indicate kind of information presented. Below is a
table of these symbols with explanation of their meaning.
Symbol
Reference
Title
Description
IEC 6417
5333
Type BF applied
part
To identify a type BF applied part
complying with IEC 60601-1
ISO 7010
M002
Refer to
instruction
manual/booklet
Indicates to read the instruction
manual/booklet before starting work or
before operating equipment or
machinery
ISO 7000
1641
Operator's
manual; operating
instructions
Indicates the need for the user to
consult the instructions for use
ISO 7000
3082
Manufacturer
Indicates the medical device
manufacturer, as defined in EU
Directives 90/385/EEC, 93/42/EEC and
98/79/EC
ISO 7000
2497
Date of
manufacture
Indicates the date when the medical
device was manufactured
ISO 7000
2493
Catalogue number
Indicates the manufacturer's catalogue
number so that the medical device can
be identified
ISO 7000
2498
Serial number
Indicates the manufacturer's serial
number so that a specific medical
device can be identified
European
Commission
European
Conformity
Conforms to European Council Directive
93/42/EEC
European
Commission
Waste Electrical
and
Electronic
Equipment
Dispose of product in accordance with
Directive 2012/19/EU
MicrUs Pro User Guide
- 11 -
System Overview
The MicrUs Pro system is a combination of ultrasound transducer and an ultrasound
beamformer in one compact handheld device. The complete system consists of:
•The MicrUs Pro device
•USB cable connecting device with controlling and visualizing device
•Laptop, tablet or smartphone with integrated USB Type-C port
For particular setup description with Android or Windows operating system please read in
corresponding chapter of this User Guide.
Getting Started with Android System
5.1. System Requirements
Please ensure that your system meets the following requirements:
•Mobile phone or tablet computer
•Android 6.0 or more recent Android operating system
•USB OTG (USB On-The-Go) port Type-C
•Quad-core (> 2 GHz) or faster CPU
•4 GB or more RAM
•32 GB or larger storage
•4000 mAh or larger capacity battery
•5-inch 1080x1920 pixels or larger display
Note: The software is used in portrait screen orientation.
5.2. Software Installation
Echo Wave A software is available on your USB key from your shipment. For updates
Note: Echo Wave A is not available from Google Play Market. It is distributed in form of
APK file. To install the software, you should allow installing applications from Unknown
Sources in your system settings.
To install Echo Wave A, copy the APK file from your USB key on your device. Find the
copied APK file using any file manager in your device storage. Run APK file to install the
Echo Wave A program.

MicrUs Pro User Guide
- 12 -
5.3. Connecting Android System
Connect the MicrUs Pro to your Android phone or tablet with USB cable included. Ensure
that Android device is well charged before you start scanning.
MicrUs Pro Android Connection Diagram
When the MicrUs Pro is not in use it is recommended to disconnect the cable
from your mobile device to save the battery charge. Keep in mind that even if
Echo Wave A app is not running the MicrUs Pro’s USB controller stays active
and consumes some energy from your battery.
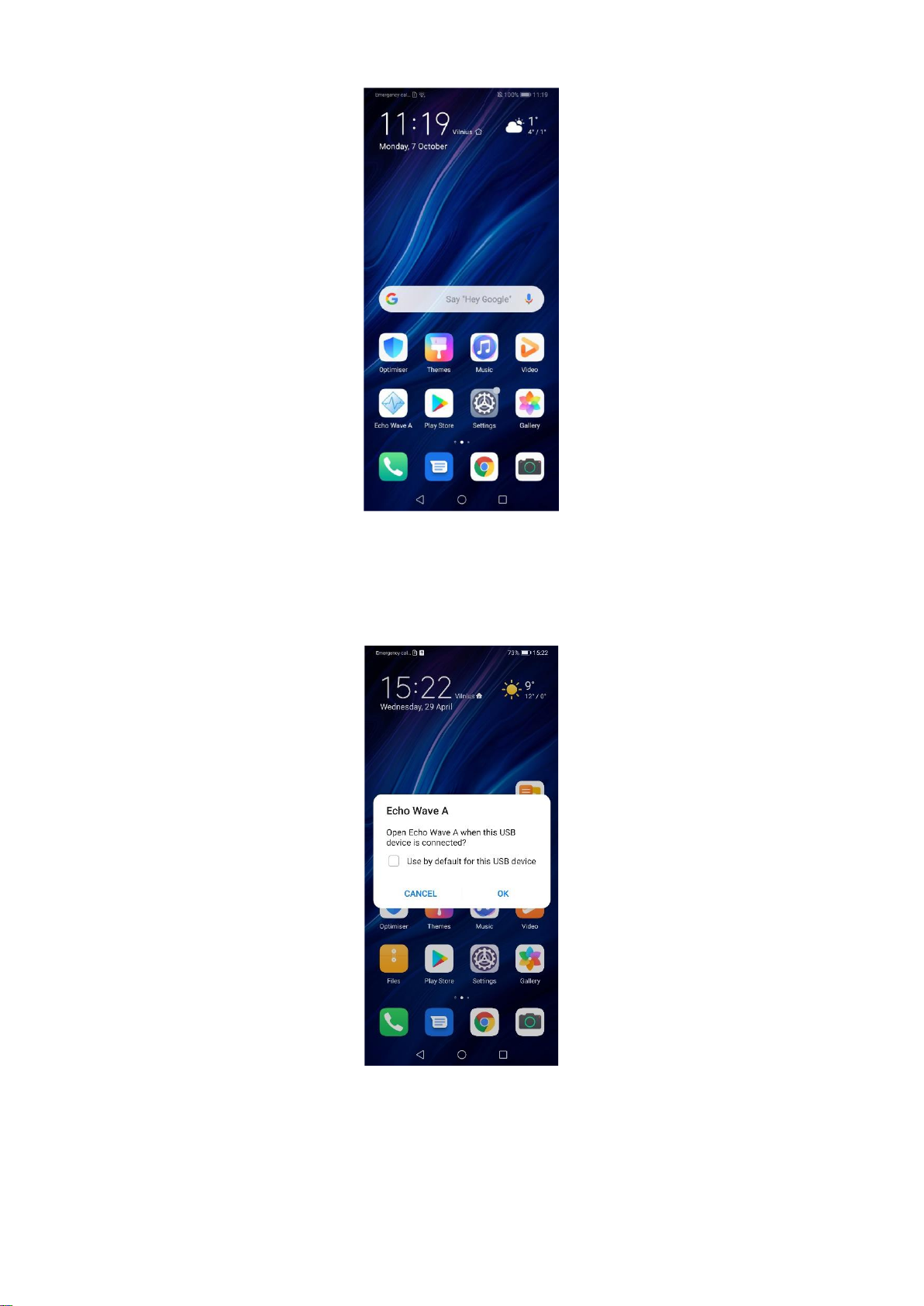
5.4. Running Echo Wave A Application
After you get installed Echo Wave A please locate its icon and tap it. See picture below
showing the typical phone screen.
NOTE
MicrUs Pro User Guide
- 13 -
Locating Echo Wave A on a Home screen.
The first time you run Echo Wave A it brings the following message, asking if you would
like to start Echo Wave A automatically when your MicrUs Pro scanner is connected. Set
check box and tap OK button if you agree.
Echo Wave A message.
Further information and step by step instructions can be found in Echo Wave A Software
User Manual located on this USB key.
MicrUs Pro User Guide
- 14 -
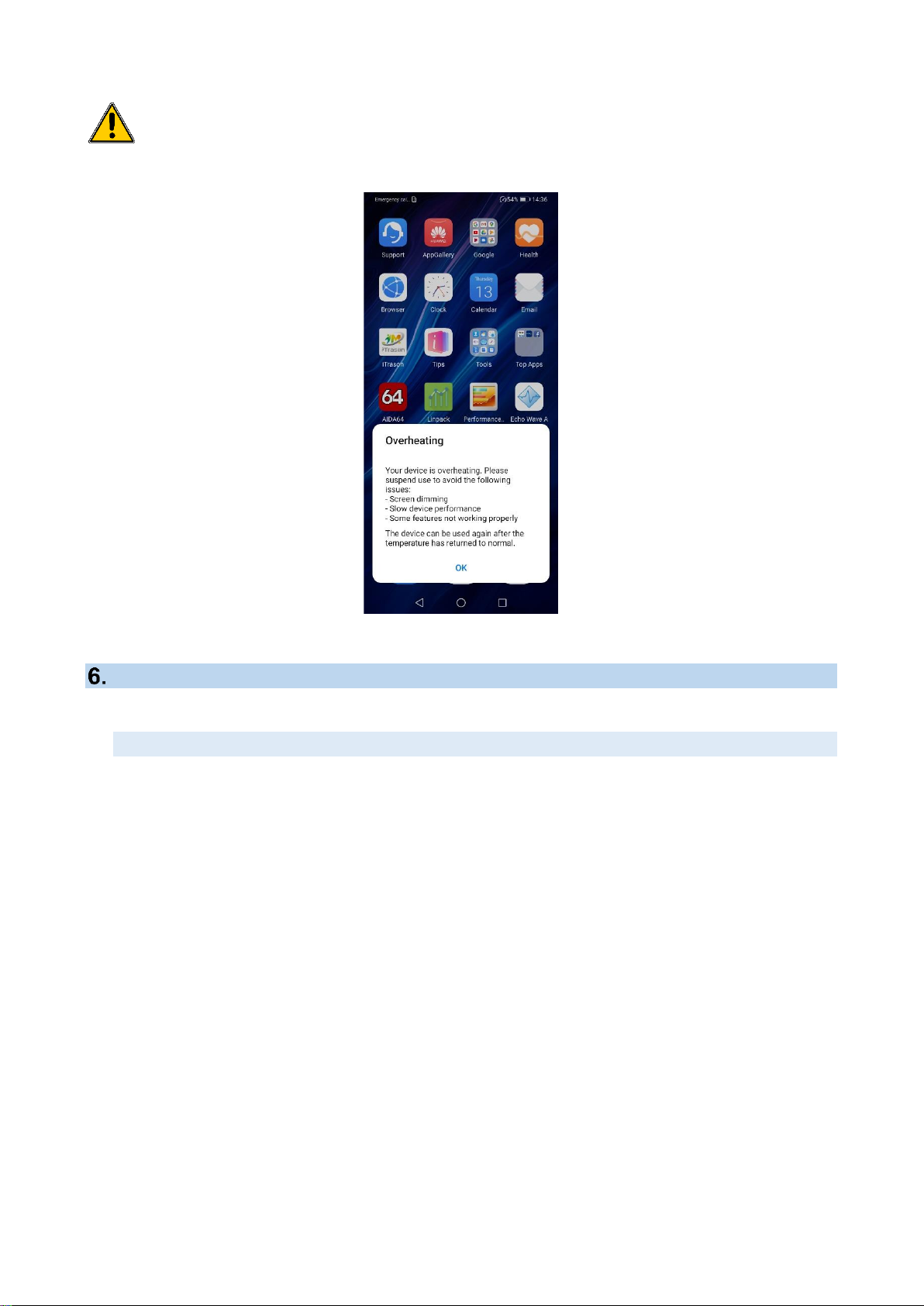
During long continuing uses of ultrasound system the mobile phone can be
internally overheated that can result with a popup message like shown below. If
that happens then allow phone to cool down switching it off or at least closing all
running applications for a reasonable amount of time.
Getting Started with Windows PC
6.1. PC Hardware Requirements
Please ensure that your PC meets the following requirements:
Hardware
•Windows(R) operating system compatible desktop, notebook or Tablet PC
•Intel chipset-based motherboard
•available USB Type-C port
•CPU i5/i7/i9 1.8 GHz or better
•2 GB RAM or more
•2 GB free hard disk space
•display with 1024x768 resolution or higher; wide viewing angles and matte covering
are recommended for better imaging; consider IPS/LPS/OLED technology, as
example
•display adapter with CUDA support (optional, for NeatView image enhancement)
•TCO certified display
•certified for medical use computer power supply
Software
•Windows 10 operating system

MicrUs Pro User Guide
- 15 -
•Microsoft .NET Framework 3.5.1
6.2. Software Installation
Ensure that the software you have supports your device. At time of writing the MicrUs Pro
is supported by:
•Echo Wave II ver.4.0.1b22 and higher
•Telemed Drivers Package ver. 2.0.1b26 and higher
1. Insert your Telemed USB key drive into USB port of your computer
2. Go to \\Software Installation\TELEMED Drivers Package ver.XXX\ folder and run
setup_tdp.exe
Installation windows will appear. Click “Next >”
MicrUs Pro User Guide
- 16 -
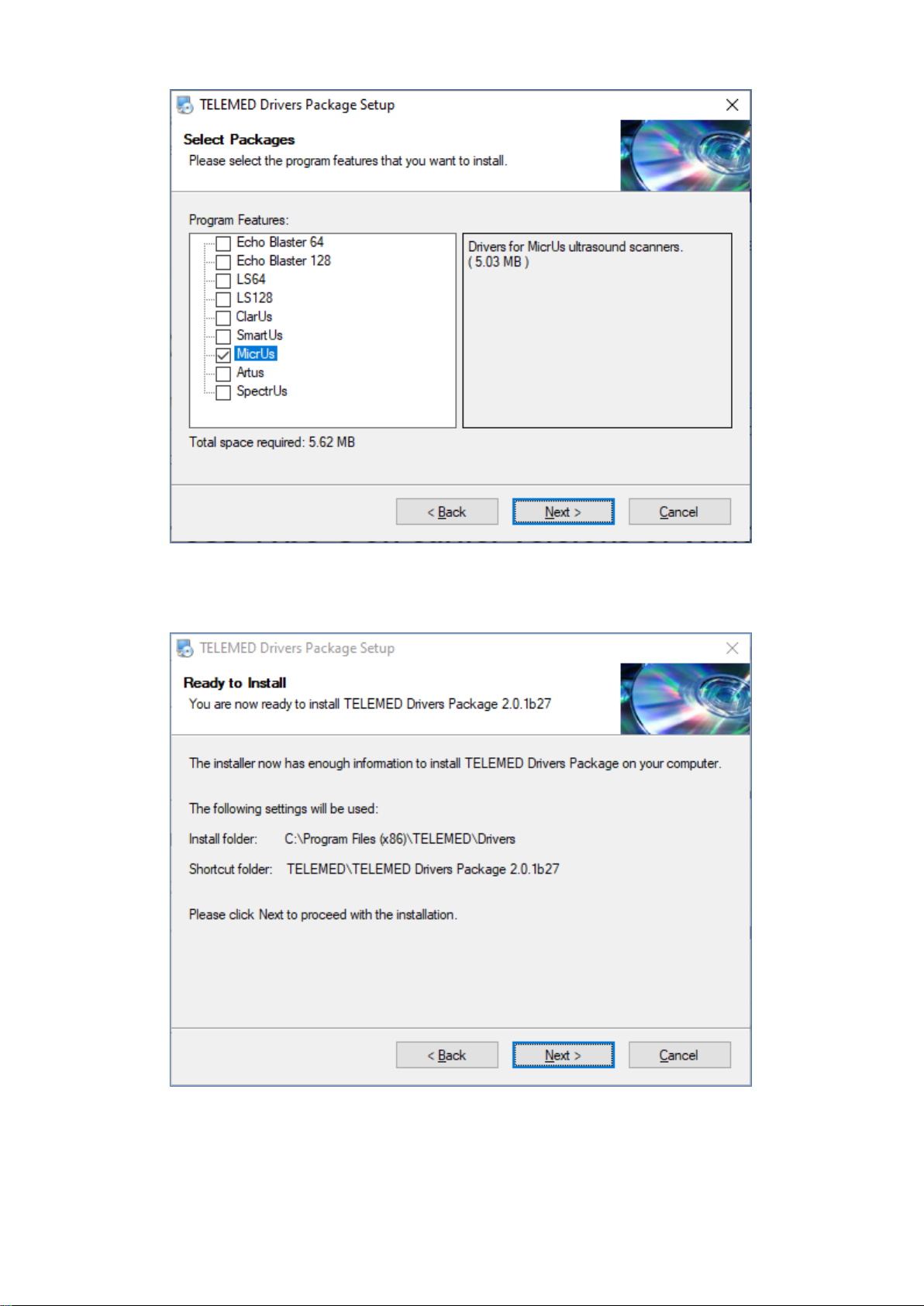
Select MicrUs checkbox, click “Next >”
Click “Next >”
MicrUs Pro User Guide
- 17 -
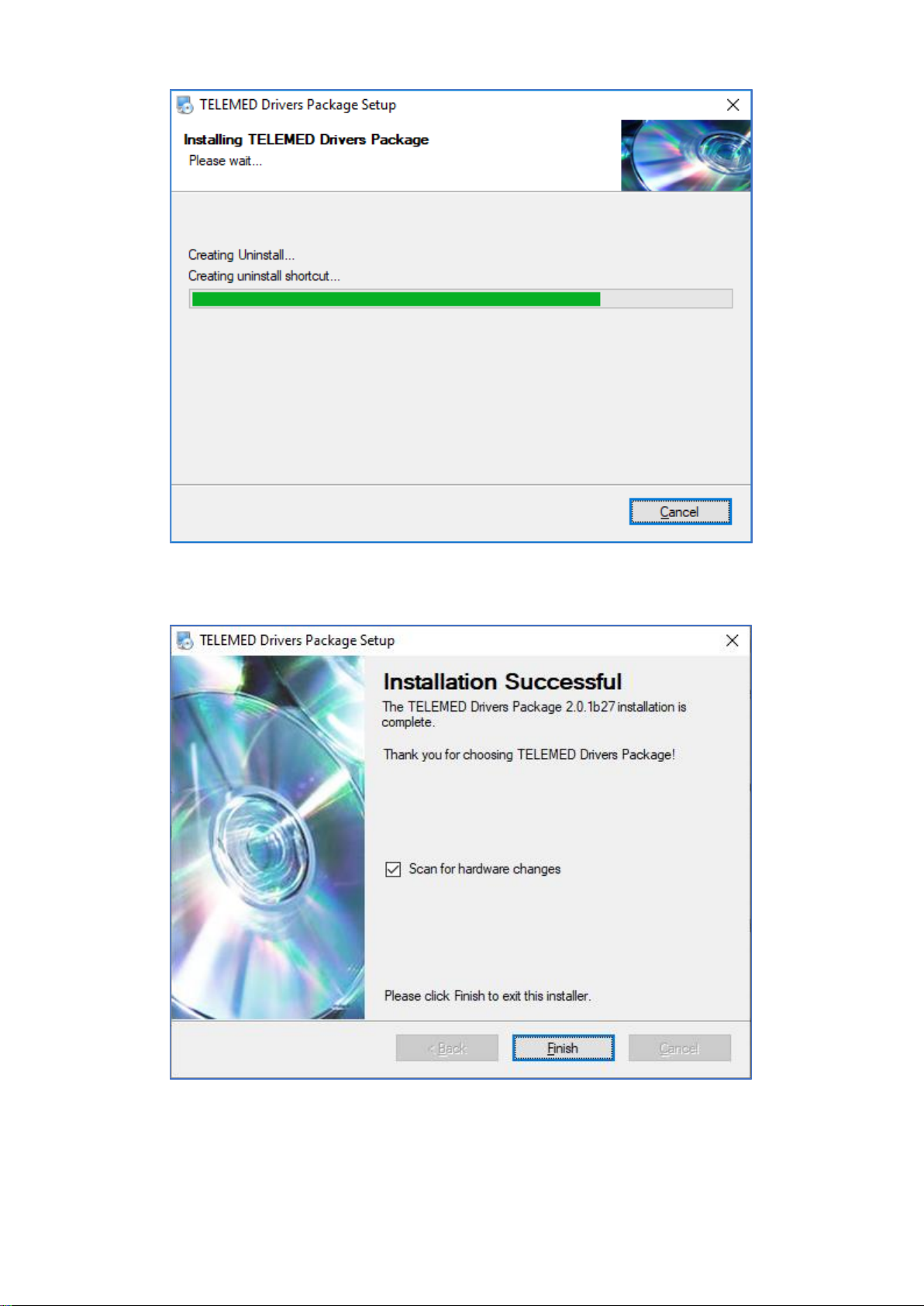
Wait until all files are copied to your hard drive…
Click “Finish”.

MicrUs Pro User Guide
- 18 -
3. Go to \\Software Installation\Echo Wave II ver.XXX\ folder and run
setup_ew2.exe
Installation windows will appear. Click “Next >”
Choose “I agree to overwrite all presets and settings”, click “Next >” button

MicrUs Pro User Guide
- 19 -
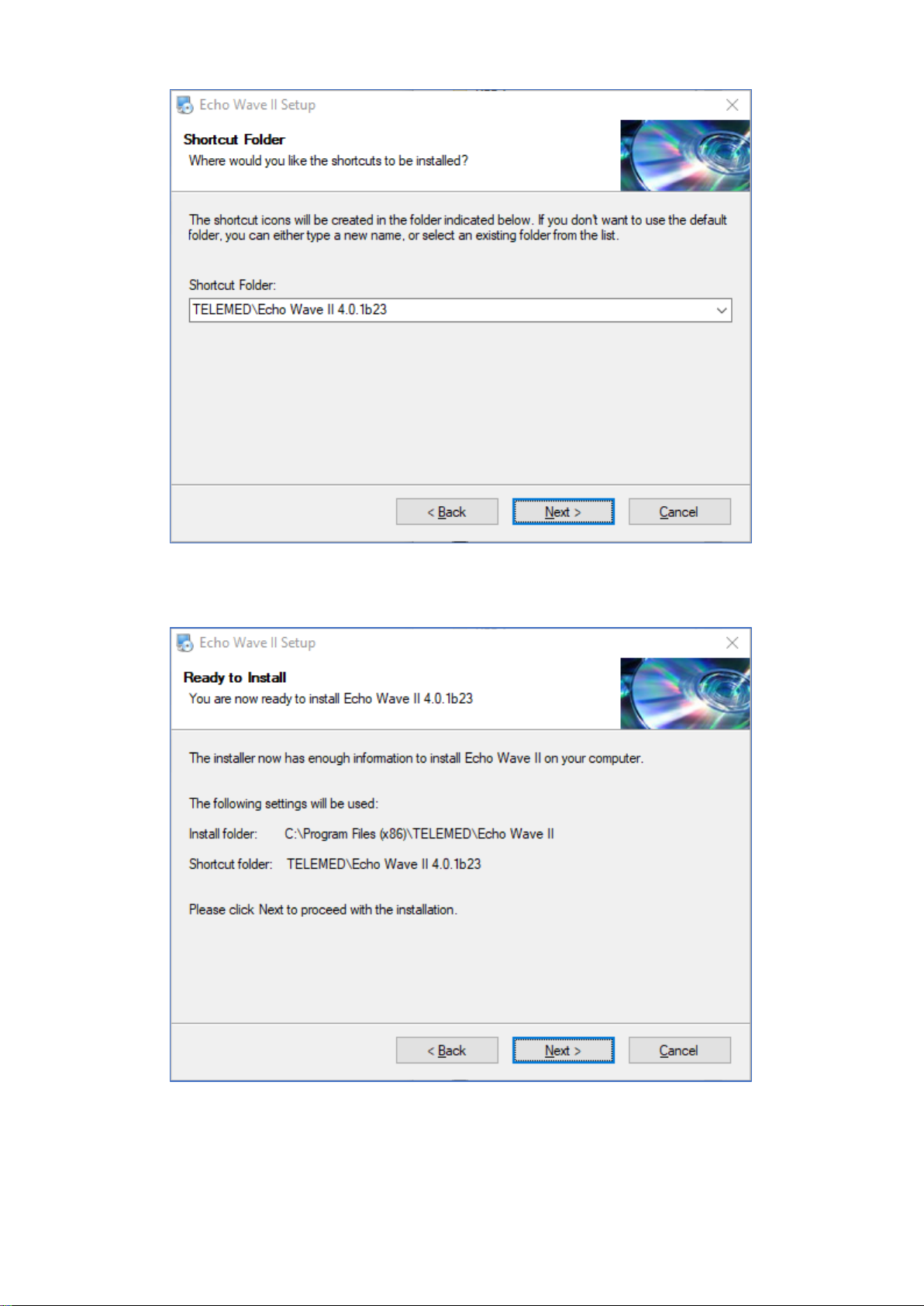
Select your region and click “Next >” button
Click “Next >” button
MicrUs Pro User Guide
- 20 -
Click “Next >” button
Click “Next >” button
Table of contents