TelePatch PM750 User manual

TelePatch™Cardiac Monitor PM750
USER MANUAL - UTM0000701-04D | January 06, 2017
TelePatch™
Cardiac Monitor PM750
User Manual
INSTRUCTIONS FOR USE
TelePatch is intended for use as prescribed by a physician who wants to follow
cardiac activity. TelePatch is not intended for diagnostic use. A physician must
review and interpret ECG findings recorded during procedure.

Have a question or need assistance?
Call Medicomp Patient Support: 800-234-3278 ext. 2370
TelePatch™Cardiac Monitor PM750
USER MANUAL - UTM0000701-04D | 01/06/20171
Table of Contents
GETTING STARTED AND INTRODUCTION .................................................................................................... 5
Notices, Cautions, and Copyrights ............................................................................................................ 5
TelePatch Cardiac Monitor User Kit.......................................................................................................... 6
Contact Us................................................................................................................................................. 9
ABOUT TELEPATCH CARDIAC MONITOR PM750 ......................................................................................... 9
INDICATIONS FOR USE .............................................................................................................................. 9
OVERVIEW............................................................................................................................................... 10
SAFETY SPECIFICATIONS AND COMPLIANCE ............................................................................................. 10
Contraindication .....................................................................................................................................10
Safety Classification ................................................................................................................................ 10
Modifications ..........................................................................................................................................10
Defibrillation ...........................................................................................................................................10
System Safety.......................................................................................................................................... 10
COMPLIANCE ..............................................................................................................................................11
Safety Classification ................................................................................................................................ 11
Radio Frequency Regulatory Compliance............................................................................................... 11
BUTTONS, ICONS AND SCREEN INDICATOR DESCRIPTION .......................................................................12
TELEPATCH SYSTEM OVERVIEW.................................................................................................................14
TelePatch Pendant Description............................................................................................................... 16
....................................................................................................................................................................18
TelePatch Smartphone Description ........................................................................................................18
Accessories to the TelePatch System...................................................................................................... 19
TelePatch Charger Cord ...................................................................................................................... 19
Connecting Charger Cord: Smartphone..............................................................................................21
TelePatch Battery Charger ..................................................................................................................22
Connecting Charger Cord: Battery Charger ........................................................................................ 23
TelePatch Electrode Patch ..................................................................................................................26
STANDARD PROCEDURE SET-UP................................................................................................................ 28
Patient Preparation................................................................................................................................. 29

Have a question or need assistance?
Call Medicomp Patient Support: 800-234-3278 ext. 2370
TelePatch™Cardiac Monitor PM750
USER MANUAL - UTM0000701-04D | 01/06/20172
Step 1 - Identify Electrode Patch Site...................................................................................................... 29
Step 2 - Prepare the Skin......................................................................................................................... 29
Step 3 - Electrode Patch Application....................................................................................................... 30
Step 4 - Turn on Pendant and Connect...................................................................................................32
Step 5 - Starting the Smartphone ...........................................................................................................34
Step 6 - Procedure Set Up....................................................................................................................... 35
Step 7 - Procedure Settings..................................................................................................................... 36
Pacemaker Settings.............................................................................................................................36
Protocol Setting................................................................................................................................... 37
Set Up..................................................................................................................................................38
Start Over............................................................................................................................................ 38
Step 8 - Initial Procedure Screens ........................................................................................................... 39
Recording Symptoms: Standard TelePatch Procedure ...........................................................................41
Manual Diary Entry .............................................................................................................................42
Audio Diary Entry ................................................................................................................................ 44
PROCEDURE SET-UP: Cable Cradle with Patient Cable ............................................................................44
Step 1 - Identify Electrode Sites..............................................................................................................44
Step 2 - Prepare the Skin......................................................................................................................... 45
Step 3 - Snap on Electrodes and Set Up Cable Cradle............................................................................. 46
Step 4 - Start Pendant............................................................................................................................. 48
Step 5 - Starting the Smartphone ...........................................................................................................48
Step 6 - Procedure Set Up....................................................................................................................... 48
Step 7 - Procedure Settings..................................................................................................................... 48
Step 8 - Initial Procedure Screens ........................................................................................................... 48
Recording Symptoms: Cable Cradle Procedure ...................................................................................... 48
PROCEDURE SET-UP: Fingertip Electrodes ................................................................................................49
Step 1 - Start Pendant............................................................................................................................. 49
Step 2 - Starting the Smartphone ...........................................................................................................49
Step 3 - Procedure Set Up....................................................................................................................... 49
Step 4 - Procedure Settings..................................................................................................................... 49
Step 5 - Initial Procedure Screens ........................................................................................................... 49

Have a question or need assistance?
Call Medicomp Patient Support: 800-234-3278 ext. 2370
TelePatch™Cardiac Monitor PM750
USER MANUAL - UTM0000701-04D | 01/06/20173
Recording Symptoms: Fingertip Electrode Procedure............................................................................ 49
COMMON QUESTIONS ............................................................................................................................... 50
How Do I Wear TelePatch System: Electrode Patch and Pendant?........................................................50
How Do I Wear TelePatch System: Pendant, Cable Cradle With Electrodes? ........................................51
Can I Shower? .........................................................................................................................................51
Tip For During Shower ............................................................................................................................51
Tip For After Shower............................................................................................................................... 51
Stress Loops ............................................................................................................................................ 52
EMERGENCY AND SUPPORT NUMBERS..................................................................................................... 52
Call 911.................................................................................................................................................... 52
PROCEDURE INFORMATION AND CAUTIONS............................................................................................ 53
TELEPATCH SYSTEM WHEN WEARING ELECTRODE PATCH ................................................................53
SHOWERING OR SWIMMING WITH TELEPATCH SYSTEM AND ELECTRODE PATCH ........................... 53
TELEPATCH SYSTEM WHEN USING CABLE CRADLE............................................................................. 54
SHOWERING OR SWIMMING WITH TELEPATCH SYSTEM AND CABLE CRADLE..................................54
SMARTPHONE / HANDSET ..................................................................................................................55
BATTERY CHARGER ............................................................................................................................. 55
PENDANT PRECAUTIONS............................................................................................................................ 56
ADVERSE REACTIONS .................................................................................................................................56
MAINTENANCE ........................................................................................................................................... 57
Cleaning the TelePatch System...............................................................................................................57
VISUAL AND AUDIBLE INDICATORS ........................................................................................................... 57
Normal Performance Indicators ............................................................................................................. 57
Error Indicators ....................................................................................................................................... 58
TROUBLESHOOTING ...................................................................................................................................59
Setup Details and Troubleshooting Overview ........................................................................................59
TelePatch System Hookup Testing Information .....................................................................................60
Quality Hookup Tests Overview.............................................................................................................. 60
The Setup/Normal Setting ...................................................................................................................... 60
Pacemakers............................................................................................................................................. 60
Lead Position Test ...................................................................................................................................61

Have a question or need assistance?
Call Medicomp Patient Support: 800-234-3278 ext. 2370
TelePatch™Cardiac Monitor PM750
USER MANUAL - UTM0000701-04D | 01/06/20174
Skin Prep Results.....................................................................................................................................61
Error Displays .......................................................................................................................................... 61
Voltage Errors after Several Attempts.................................................................................................... 62
Lead Position Tests..................................................................................................................................62
Lead Test Error........................................................................................................................................ 62
High/Low Voltage Notifications ..............................................................................................................64
LOW VOLTAGE IN CHANNEL A ............................................................................................................64
LOW VOLTAGE IN CHANNEL B ............................................................................................................64
HIGH VOLTAGE IN CHANNEL A............................................................................................................ 64
HIGH VOLTAGE IN CHANNEL B............................................................................................................65
SAMPLE SCREEN WARNINGS .....................................................................................................................65
Screen Warnings ..................................................................................................................................... 65
Unable to find Pendant........................................................................................................................... 65
Pendant is Turned Off ............................................................................................................................. 66
Procedure Has Ended.............................................................................................................................. 66
Start Procedure Exceptions..................................................................................................................... 66
Selecting a Pendant............................................................................................................................. 66
SAFE BATTERY USE AND DISPOSAL............................................................................................................ 66
Safely Loading the Pendant Battery........................................................................................................ 67
Battery Maintenance ..............................................................................................................................69
Disposal of Rechargeable Lithium Ion Batteries .....................................................................................69
EXPLANATION OF MARKINGS....................................................................................................................69
EXPECTED LIFE ............................................................................................................................................ 71
PENDANT SPECIFICATIONS.........................................................................................................................72
Guidance and Manufacturer’s Declaration - Electromagnetic Emissions ................................................73
Recommended separation distances between portable and mobile RF communications equipment and
the PM750................................................................................................................................................... 75

Have a question or need assistance?
Call Medicomp Patient Support: 800-234-3278 ext. 2370
TelePatch™Cardiac Monitor PM750
USER MANUAL - UTM0000701-04D | 01/06/20175
GETTING STARTED AND INTRODUCTION
Notices, Cautions, and Copyrights
CAUTION: FEDERAL LAW RESTRICTS TELEPATCH CARDIAC MONITOR PM750 FOR SALE
BY, OR ON THE ORDER OF, A LICENSED MEDICAL PRACTITIONER. THE DATA
OBTAINED FROM THE PENDANT IS FOR THE REVIEW BY A PHYSICIAN. IT IS
RECOMMENDED THAT A PHYSICIAN OVERREAD THE RESULTS.
THIS TELEPATCH CARDIAC MONITOR PM750 IS NOT INTENDED FOR USE BY USERS WHO ARE
UNABLE TO ACTIVATE THE SYMPTOM BUTTON WHEN THEY ARE EXPERIENCING A SYMPTOM.
USERS SHOULD BE SUPERVISED IF UNABLE TO ACTIVATE THE SYMPTOM BUTTON ON THEIR
OWN.
TelePatch™Cardiac Monitor PM750 is a product of Medicomp, Inc.
Medicomp, Inc. is the manufacturer and provider of TelePatch products and services. TelePatch
is a trademark of Medicomp, Inc. ©2016 Medicomp, Inc. All rights reserved.
COPYRIGHT MEDICOMP, INC. 2016 ALL RIGHTS RESERVED. NO PART OF THIS PUBLICATION MAY
BE REPRODUCED OR DISTRIBUTED IN ANY FORM OR BY MEANS WITHOUT PRIOR WRITTEN
PERMISSION FROM MEDICOMP, INC.

Have a question or need assistance?
Call Medicomp Patient Support: 800-234-3278 ext. 2370
TelePatch™Cardiac Monitor PM750
USER MANUAL - UTM0000701-04D | 01/06/20176
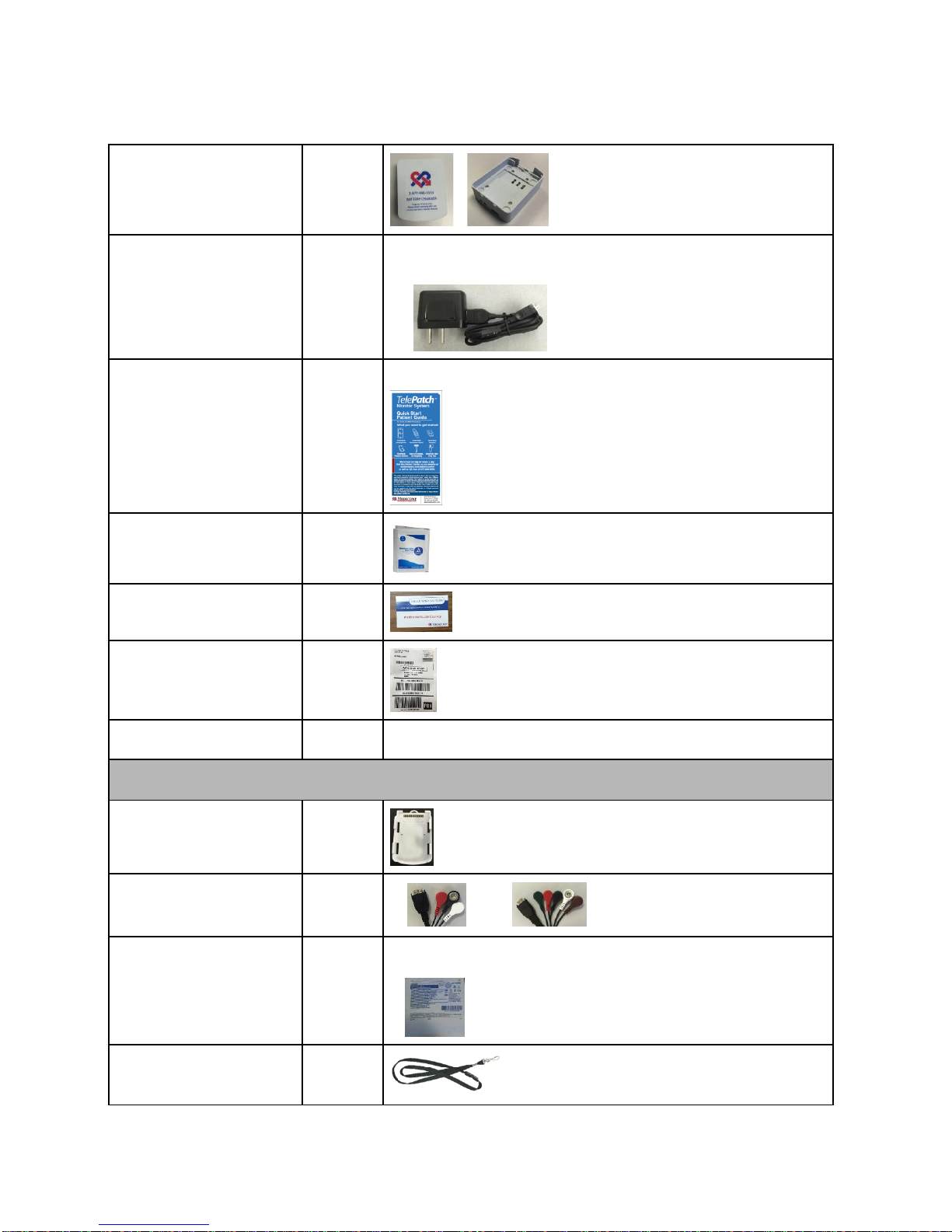
TelePatch Cardiac Monitor User Kit
The standard TelePatch Cardiac Monitor PM750 kit comes with:
1. TelePatch Pendant
2. Electrode Patch with Cradle
3. Rechargeable Batteries
4. TelePatch Smartphone
5. Battery Charger
6. Charger Cord for both Smartphone and Battery Charger
7. Electrode Prep Pad
8. Quick Start Patient Guide
9. Survey Invitation
10. Return Envelope with Pre-paid Shipping Label
TelePatch Cardiac Monitor Kit

Have a question or need assistance?
Call Medicomp Patient Support: 800-234-3278 ext. 2370
TelePatch™Cardiac Monitor PM750
USER MANUAL - UTM0000701-04D | 01/06/20177
TELEPATCH KIT CONTENTS
Item
Quantity
Picture
TelePatch
Pendant
1
Electrode Patch
with Cradle*
1 - 4
*Kit will have one or the other patch style, single or double is based
on procedure prescribed by physician
Rechargeable Battery
2
TelePatch
Smartphone*
1
*Holter procedures do not require a Smartphone
Have a question or need assistance?
Call Medicomp Patient Support: 800-234-3278 ext. 2370
TelePatch™Cardiac Monitor PM750
USER MANUAL - UTM0000701-04D | 01/06/20178
Battery Charger
1
Charger Cord
for Smartphone
and Battery Charger
1
Quick Start
Patient Guide
1
Electrode Prep Pad
1
Survey Invitation
1
Return Envelope with
Pre-paid Shipping Label
1
Legal Booklet
1
PICTURE TO BE ADDED
These items are not standard in TelePatch Kit. By physician request only
Cable Cradle
1
Cable
3-lead or 5-lead
1
Electrodes
(3-5 per pouch)
1- 4
Breakaway Lanyard
1

Have a question or need assistance?
Call Medicomp Patient Support: 800-234-3278 ext. 2370
TelePatch™Cardiac Monitor PM750
USER MANUAL - UTM0000701-04D | 01/06/20179
Contact Us
Contact Medicomp, Inc. for any issues concerning the TelePatch product such as:
• General questions about our product
• Product safety
• Safe disposal of component parts
• Return of product
Contact information
Medicomp, Inc.
600 Atlantis Rd.
Melbourne, FL, 32904
Website: www.medicompinc.com
ABOUT TELEPATCH CARDIAC MONITOR PM750
INDICATIONS FOR USE
The TelePatch Cardiac Monitor PM750, is a pager-sized, handheld or patient worn device
designed specifically to record and transmit ambulatory ECG signals. The device is designated as
Rx only, to be worn by infants to adults of all ages. The device can be worn for days or weeks, as
it is intended for use by patients who are experiencing symptoms that are transient and
infrequent in nature.

Have a question or need assistance?
Call Medicomp Patient Support: 800-234-3278 ext. 2370
TelePatch™Cardiac Monitor PM750
USER MANUAL - UTM0000701-04D | 01/06/201710
The TelePatch Cardiac Monitor System
OVERVIEW
Users of the TelePatch System should be able to activate the Symptom button unassisted or be
supervised and assisted. The TelePatch System can be worn by users weighing less than 10kg.
TelePatch is intended for use as prescribed by a physician who wants to follow cardiac activity.
A physician must review and interpret ECG findings recorded during procedure. The TelePatch
System is not intended for diagnostic use.
SAFETY SPECIFICATIONS AND COMPLIANCE
Contraindication
There are no potential adverse effects of the TelePatch Cardiac Monitor, PM750 on health.
Safety Classification
In accordance with IEC 60601-1 Third Edition Am 1: 2012:
●This equipment is designed to be operated with one 3.7V 440mAh 1.62Wh lithium ion battery, and under no circumstances shall
power be supplied in any other manner.
●Type BF equipment.
●Rated for Continuous Operation.
●Ordinary Equipment. Enclosed equipment. The device is protected to IP55 as required by the standard.
●This equipment shall not be used in the presence of a flammable anesthetic mixture with air or with oxygen or nitrous oxide, or
flammable cleaning agents.
●Equipment with an Applied Part, specifically designed for applications where a Conductive Connection is made to the Patient, but
not directly to the heart.
●The equipment requires no adjustment.
●Operating and Storage Humidity: 10% to 95%, non-condensing
●Operating Temperature: 0°C to 45°C (32°F to 284°F).
●Storage Temperature: -15°C to 60°C (5°F to 140°F).
●TelePatch System Shipment: Temperature limitation for shipment: -15°C to 60°C (5°F to 140°F).
●Atmospheric Pressure: Operating: 700 hPa to 1060 hPa; Storage/Transport: Not Applicable to TelePatch System
The equipment contains no user-serviceable parts. It shall be serviced only by Medicomp, Inc. Unauthorized repairs of the equipment will void
the warranty.
Modifications
For continued safety, equipment should not be modified in any manner and must be used only as indicated.
Defibrillation
The external parts of the equipment may provide a source of the defibrillation voltage if it is not removed from the patient during defibrillation.
Due to the small size of the unit and patient connectors, the cable or connector may break down and cause the defibrillation voltage to be
shunted and make it less effective for the patient. The unit and cable MUST be removed prior to defibrillation.
System Safety
Additional equipment connected to medical electrical equipment must comply with the respective IEC or ISO standards (e.g. IEC 60950 for data
processing equipment). Furthermore all configurations shall comply with the requirements for medical electrical systems (see IEC 60601-1-1 or
clause 16 of the 3Ed. of IEC 60601-1, respectively). Anybody connecting additional equipment to medical electrical equipment configures a

Have a question or need assistance?
Call Medicomp Patient Support: 800-234-3278 ext. 2370
TelePatch™Cardiac Monitor PM750
USER MANUAL - UTM0000701-04D | 01/06/201711
medical system and is therefore responsible that the system complies with the requirements for medical electrical systems. Attention is drawn
to the fact that local laws take priority over the above mentioned requirements. If in doubt, consult your local representative or the technical
service department
WARNINGS
USE THE TELEPATCH SYSTEM ONLY WITH THE LEADS, ELECTRODES, AND ACCESSORIES RECOMMENDED BY MEDICOMP. USE OF
OTHER ACCESSORIES MAY ADVERSELY AFFECT THE PERFORMANCE OF THE DEVICE OR MAY RESULT IN STRONGER
ELECTROMAGNETIC EMISSIONS OR REDUCE THE ELECTROMAGNETIC IMMUNITY OF TELEPATCH CARDIAC MONITOR PM750
CONDUCTIVE PARTS OF ELECTRODES AND ASSOCIATED CONNECTORS FOR TYPE BF OR CF APPLIED PARTS, INCLUDING NEUTRAL
ELECTRODE, SHOULD NOT CONTACT OTHER CONDUCTIVE PARTS, INCLUDING EARTH.
USERS WHO ARE WEARING NEUROSTIMULATING PENDANTS CANNOT BE SET UP WHILE THAT DEVICE IS TURNED ON. THE
OPERATION OF THESE PENDANTS INTERFERES WITH THE TELEPATCH’S ABILITY TO ACQUIRE THE ECG SIGNAL. IF ALLOWED THESE
DEVICES SHOULD BE TURNED OFF WHILE THE PATIENT IS WEARING TELEPATCH.
DO NOT USE THE TELEPATCH IN COMBINATION WITH EXTERNAL CARDIAC DEFIBRILLATORS OR HIGH FREQUENCY SURGICAL
EQUIPMENT.
PORTABLE AND MOBILE RF COMMUNICATIONS EQUIPMENT CAN AFFECT MEDICAL ELECTRICAL EQUIPMENT. THIS PENDANT
SHOULD NOT BE USED ADJACENT TO OR STACKED WITH OTHER EQUIPMENT.
LOAD ONLY 3.7V LITHIUM ION BATTERIES DELIVERED IN THE TELEPATCH KIT INTO THE TELEPATCH CARDIAC MONITOR BATTERY
COMPARTMENT.
LEAD FAILURES ARE DETECTED BY A 10 MV PEAK, 50% DUTY CYCLE RECTANGULAR PULSE, WHICH IS APPLIED TO EACH PATIENT
ELECTRODE CONNECTION THROUGH A 4.9MOHM RESISTOR AT A RATE OF 15 HZ WITH RESPECT TO THE SYSTEM GROUND.
THIS IS A PRESCRIBED MEDICAL DEVICE, NOT A TOY, INFANTS AND CHILDREN MUST BE SUPERVISED.
WARNING: CHOKING HAZARD –ADULT SUPERVISION REQUIRED
IN THE EVENT OF A DAMAGED PENDANT, DISCONTINUE USE AND CALL MEDICOMP PATIENT SUPPORT: 800-234-3278 EXT. 2370,
FOR RETURN AND REPLACEMENT.
COMPLIANCE
Safety Classification
In accordance with IEC 60601-1 Third Edition Am 1:2012 :
Conformance to Standards –non-clinical testing demonstrated conformance to voluntary safety IEC 60601-1 and to IEC 60601-1-2-2001 Class B
Medicomp, Inc.’s Quality System conforms to 21 CFR 820 and ISO 13485:2003
Radio Frequency Regulatory Compliance
Conformance to Standards
Non-clinical testing demonstrated conformance to voluntary safety IEC 60601-1 and to IEC 60601-1-2-2001 Class B
Medicomp, Inc.’s Quality System conforms to 21 CFR 820 and ISO 13485:2003
This Pendant contains transmitter module FCC ID:
Model Name: TAS0000700
FCC ID: 2AGDTPM750
IC account number/IC company number: 21061-PM750
CAN ICES-3 (B)/NMB-3(B)”
This Pendant complies with part 15 of the FCC Rules. Operation is subject to the following two conditions: (1) This Pendant may not cause
harmful interference, and (2) this Pendant must accept any interference received, including interference that may cause undesired operation.
Part 15 Clause 15.105
Note: This equipment has been tested and found to comply with the limits for a Class B digital Pendant, pursuant to part 15 of the FCC Rules.
These limits are designed to provide reasonable protection against harmful interference in a residential installation. This equipment generates
uses and can radiate radio frequency energy and, if not installed and used in accordance with the instructions, may cause harmful interference
to radio communications. However, there is no guarantee that interference will not occur in a particular installation. If this equipment does
cause harmful interference to radio or television reception, which can be determined by turning the equipment off and on, the user is
encouraged to try to correct the interference by one or more of the following measures:
—Reorient or relocate the receiving antenna.
—Increase the separation between the equipment and receiver.
—Connect the equipment into an outlet on a circuit different from that to which the receiver is connected.
Have a question or need assistance?
Call Medicomp Patient Support: 800-234-3278 ext. 2370
TelePatch™Cardiac Monitor PM750
USER MANUAL - UTM0000701-04D | 01/06/201712
—Consult the dealer or an experienced radio/TV technician for help.
Part 15 Clause 15.21
Changes or modifications not expressly approved by the party responsible for compliance could void the user's authority to operate the
equipment
This Pendant complies with Industry Canada license-exempt RSS standard(s). Operation is subject to the following two conditions: (1) this
Pendant may not cause interference, and (2) this Pendant must accept any interference, including interference that may cause undesired
operation of the Pendant.
Le présent appareil est conforme aux CNR d'Industrie Canada applicables aux appareils radio exempts de licence. L'exploitation est autorisée aux
deux conditions suivantes: (1) l'appareil ne doit pas produire de brouillage, et (2) l'utilisateur de l'appareil doit accepter tout brouillage
radioélectrique subi, même si le brouillage est susceptible d'en compromettre le fonctionnement. »
Under Industry Canada regulations, this radio transmitter may only operate using an antenna of a type and maximum (or lesser) gain approved
for the transmitter by Industry Canada. To reduce potential radio interference to other users, the antenna type and its gain should be so chosen
that the equivalent isotropically radiated power (e.i.r.p.) is not more than that necessary for successful communication.
Conformément à la réglementation d'Industrie Canada, le présent émetteur radio peut fonctionner avec une antenne d'un type et d'un gain
maximal (ou inférieur) approuvé pour l'émetteur par Industrie Canada. Dans le but de réduire les risques de brouillage radioélectrique à
l'intention des autres utilisateurs, il faut choisir le type d'antenne et son gain de sorte que la puissance isotrope rayonnée équivalente (p.i.r.e.) ne
dépasse pas l'intensité nécessaire à l'établissement d'une communication satisfaisante.
BUTTONS, ICONS AND SCREEN INDICATOR DESCRIPTION
Below is a detailed description of TelePatch Icons and Screen Indicators.
USER GUIDE DESCRIPTIONS FOR SCREEN INDICATORS AND ICONS ARE FOR USE WITH THE
TELEPATCH SMARTPHONE APPLICATION ONLY AND NOT FOR NORMAL CELLULAR PHONE
USAGE.
TELEPATCH APPLICABLE BUTTONS
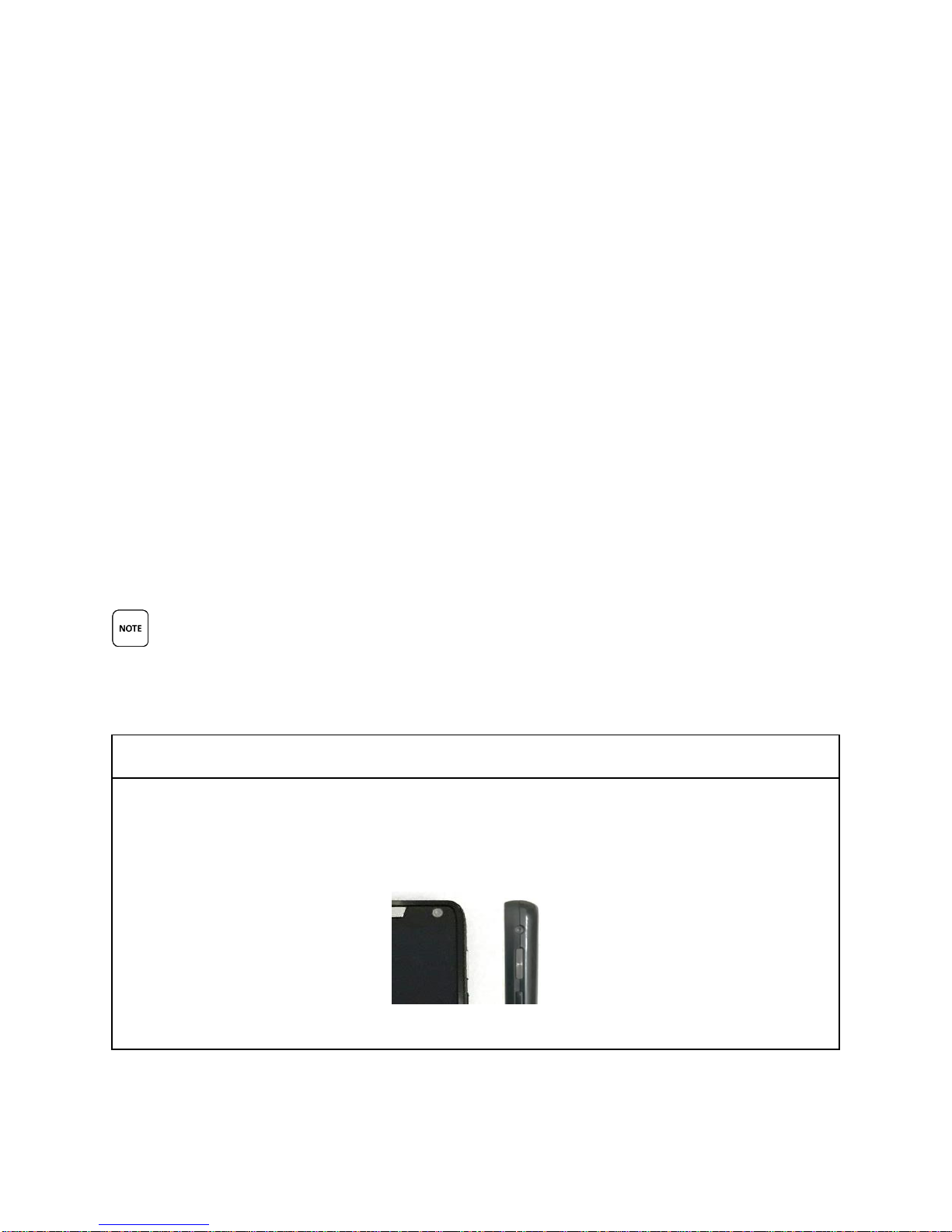
The Smartphone POWER Button
This is the Smartphone POWER button. It is located on the top, RIGHT side of the
Smartphone.
Front View Side View

Have a question or need assistance?
Call Medicomp Patient Support: 800-234-3278 ext. 2370
TelePatch™Cardiac Monitor PM750
USER MANUAL - UTM0000701-04D | 01/06/201713
The Pendant POWER Button
This is the Pendant POWER button. It is located on the FACE of the Pendant.
The Pendant Symptom Button
This is the Pendant Symptom button. It is located in the center of the FACE of the Pendant.
Users push the Symptom* button to manually initiate the recording of symptoms that they
may be experiencing. (*Symptom capture can also be initiated on the Smartphone)
The Smartphone Needs Charging Indicator
Smartphone will have red flashing LED at top of Smartphone when battery is low. This LED
will stop flashing when plugged into the Charger Cord and Smartphone is charging.
The Smartphone Charged Light Indicator
There is no light indicator showing Smartphone is charging or full charged. Smartphone
battery percentage will read 100%.
TELEPATCH APPLICABLE TOUCH KEYS - Smartphone
Start Procedure
Initiates procedure for the TelePatch system.
Symptom Button
Press when experiencing a symptom.
FREQUENT SCREEN ICONS AND INDICATORS
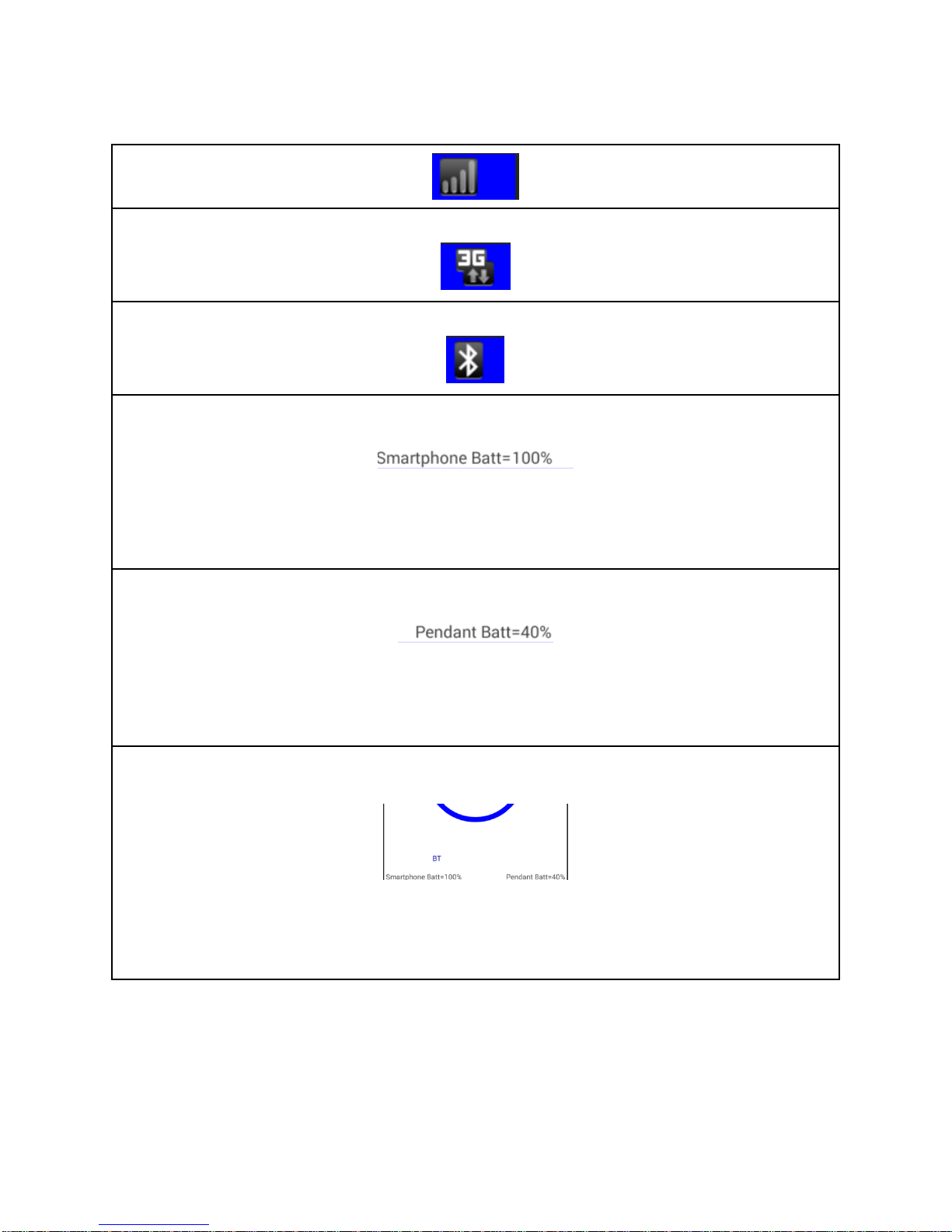
Cell Network Strength Icon
Have a question or need assistance?
Call Medicomp Patient Support: 800-234-3278 ext. 2370
TelePatch™Cardiac Monitor PM750
USER MANUAL - UTM0000701-04D | 01/06/201714
3G Connectivity Icon
Bluetooth Connectivity Icon
The Smartphone Battery Strength Indicator
This is the Smartphone Battery Strength Indicator in percentages. This is located in the lower
left corner of the Smartphone screen once Bluetooth communication has been set up. In this
example, the Smartphone battery strength is 100 percent.
Pendant Battery Strength Indicator
This is the Pendant Battery Strength Indicator in percentages. This is located in the lower right
corner of the Smartphone screen once Bluetooth communication has been set up. In this
example, the Pendant battery strength is 40 percent.
The Bluetooth Communication Indicator
This is the Bluetooth communication Indicator. Once Procedure Setup has been completed
and communication with the Pendant has been established, ' BT' will appear in the bottom
left side of the Smartphone screen.
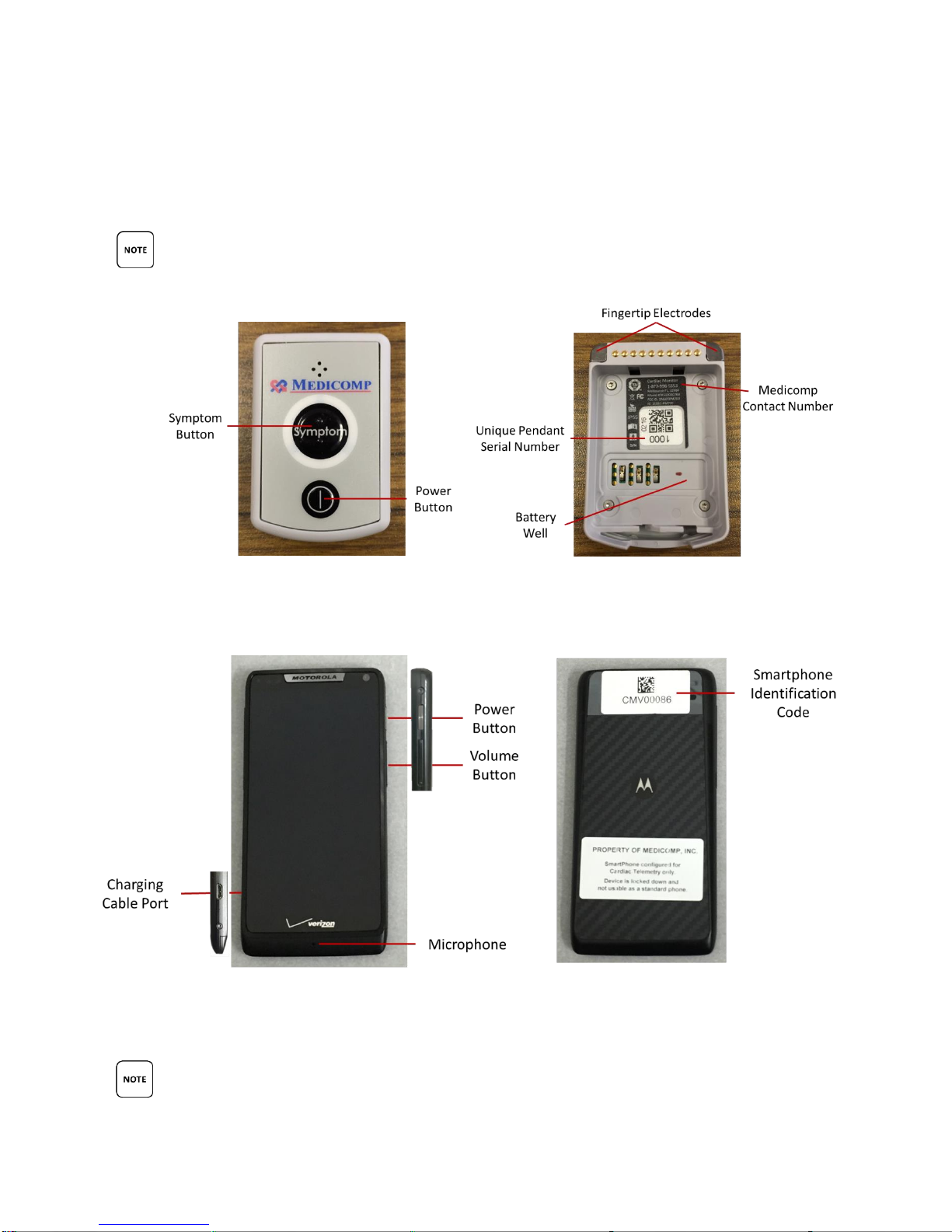
TELEPATCH SYSTEM OVERVIEW
Have a question or need assistance?
Call Medicomp Patient Support: 800-234-3278 ext. 2370
TelePatch™Cardiac Monitor PM750
USER MANUAL - UTM0000701-04D | 01/06/201715
Below is a graphical overview of the TelePatch System. Detailed description of icons, buttons,
and screen indicators are in previous section.
KEY DESCRIPTIONS PROVIDED BELOW ARE FOR USE WITH THE TELEPATCH APPLICATION
ONLY. KEY DESCRIPTIONS ARE NOT INTENDED FOR NORMAL CELLULAR PHONE USAGE.
Front of TelePatch Pendant Back of TelePatch Pendant
Front of TelePatch Smartphone Back of TelePatch Smartphone
PENDANT AND SMARTPHONE PICTURED ARE NOT ACTUAL SIZE.

Have a question or need assistance?
Call Medicomp Patient Support: 800-234-3278 ext. 2370
TelePatch™Cardiac Monitor PM750
USER MANUAL - UTM0000701-04D | 01/06/201716
TELEPATCH CARDIAC MONITOR DESCRIPTION
TelePatch Pendant Description
Below is an overview of the TelePatch Pendant, which is part of the TelePatch Cardiac Monitor
System. The TelePatch Pendant is very light-weight. It is a light gray, pager-sized ECG data
collecting Pendant.
The Pendant SYMPTOM Button
The TelePatch Pendant has a Symptom* button which is located in the center of the face of the
Pendant. (*Symptoms can also be initiated on the Smartphone screen). The Symptom button
has two raised bars so it can be distinguished from power button by touch.
The Pendant POWER Button
The Pendant POWER button is located on the face of the Pendant, below the Symptom button.

Have a question or need assistance?
Call Medicomp Patient Support: 800-234-3278 ext. 2370
TelePatch™Cardiac Monitor PM750
USER MANUAL - UTM0000701-04D | 01/06/201717
Fingertip Electrode Accessory
The fingertip electrode is built into the TelePatch pendant. Fingertip electrodes are used when
the patient cannot tolerate wearing the Electrode Patch or individual electrode patches.
Fingertip electrode procedures should be performed only after consulting your physician.
Detailed directions about fingertip electrode procedures begin on page 49.
TelePatch Battery
Bottom and top views of the TelePatch lithium ion battery
TelePatch Pendant operates on a rechargeable, lithium ion battery. The battery locks into the
battery well of the back of the TelePatch Pendant.

Have a question or need assistance?
Call Medicomp Patient Support: 800-234-3278 ext. 2370
TelePatch™Cardiac Monitor PM750
USER MANUAL - UTM0000701-04D | 01/06/201718
THE RECHARGEABLE BATTERY PROVIDED WITH THE TELEPATCH SYSTEM IS NOT TO BE
DISCARDED IN HOUSEHOLD WASTE. IF A BATTERY GETS DAMAGED CONTACT MEDICOMP, INC.:
877-996-5553
TelePatch Pendant
Battery Well Battery properly inserted in Battery Well
Detailed directions to change and charge the battery properly are found on page 25 or page 68.
IT IS VERY IMPORTANT TO TURN THE PENDANT OFF BEFORE CHANGING BATTERY,
OTHERWISE DATA MAY BE LOST. THE POWER BUTTON IS LOCATED ON THE FRONT FACE OF THE
PENDANT BELOW THE SYMPTOM BUTTON.
TelePatch Smartphone Description
Below is an overview of the TelePatch
Smartphone, which is part of the
TelePatch System.

Have a question or need assistance?
Call Medicomp Patient Support: 800-234-3278 ext. 2370
TelePatch™Cardiac Monitor PM750
USER MANUAL - UTM0000701-04D | 01/06/201719
Accessories to the TelePatch System
TelePatch Charger Cord
The TelePatch Charger Cord is compliant for use with the Smartphone and the Battery Charger.
Both are components of the TelePatch System. The Charger Cord is a two piece device:
MicroUSB Cable AC Power Supply
MicroUSB Cable
SKN6430A / S13239-0403532
AC Power Supply
SPN5504A / S004ASU0510085
Table of contents
Popular Medical Equipment manuals by other brands

Gima
Gima TOBI MANUALE manual

Nonin
Nonin 8000AA quick guide

Mobility Research
Mobility Research LITEGAIT II Operators & service manual

Hydrogen For Health
Hydrogen For Health H2Pro Series operating instructions

bort medical
bort medical EpiBasic Sport Instructions for use

Beurer
Beurer EM 41 Instructions for use