ToronTek E400 User manual

User Instructions
This Manual is written and compiled in accordance with the council directive MDD93/42/EEC for
medical devices and harmonized standards. In case of modifications and software upgrades, the
information contained in this document is subject to change without notice.
The Manual describes, in accordance with the Pulse Oximeter’s features and requirements, the main
structure, functions, specifications, correct methods for transportation, installation, usage, operation,
repair, maintenance and storage, etc. as well as the safety procedures to protect both the user and
equipment. Refer to the respective chapters for details.
Please read the User Manual carefully before using this product. The User Manual which describes the
operating procedures should be followed strictly. Failure to follow the User Manual may cause
measuring abnormality, equipment damage and human injury. The manufacturer is NOT responsible for
the safety, reliability and performance issues and any monitoring abnormalities, human injury or
equipment damage due to users' negligence of the operation instructions. The manufacturer’s warranty
service does not cover such faults.
Regrettably, owing to the forthcoming renovation, the specific products you received may not be totally
in accordance with the description of this User Manual.
This product is a medical device and can be used repeatedly.
WARNING:
Discomfort or pain can occur if the device is used ceaselessly, especially for
microcirculation barrier patients. It is recommended that the sensor should not be applied
to the same finger for longer than 2 hours.
For special patients, there should be a more prudent inspection of the placing process. The
device should not be clipped on an edema or tender tissue.
The infrared light emitted from the device, though invisible, is harmful to the eyes. Avoid
staring at it.
The patient cannot use nail enamel or other makeup.
The patient’s fingernails must be clipped short.
Please refer to the related content about clinical restrictions and caution.
This device is not intended for treatment.
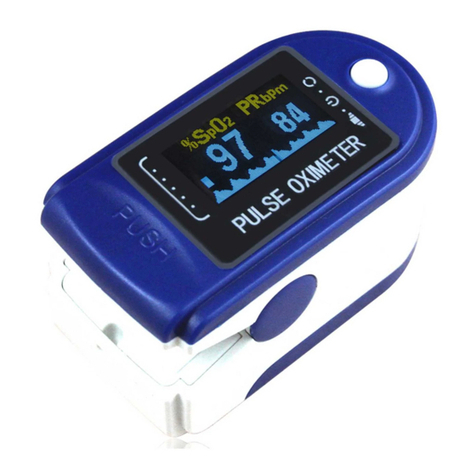
TORONTEK-E400 Pulse Oximeter

Table of Contents
1. Safety..................................................................................................................................................... 1
1.1 .Instructions for Safe Operations ......................................................................................................1
1.2. Warning............................................................................................................................................ 1
1.3. Hazards ............................................................................................................................................ 1
2. Overview..............................................................................................................................................2
2.1. Features............................................................................................................................................ 2
2.2. Major Applications and Scope of Application .................................................................................3
2.3. Environment requirements............................................................................................................... 3
3. Principle................................................................................................................................................3
4. Technical specifications ........................................................................................................................4
4.1. Main performance............................................................................................................................4
4.2. Main parameters............................................................................................................................... 4
5. Installation............................................................................................................................................5
5.1. View of Front Panel ........................................................................................................................5
5.2. Installing the Wrist Strap.................................................................................................................5
5.3. USB Port..........................................................................................................................................5
5.4. Accessories.......................................................................................................................................5
6. Operating guide.................................................................................................................................... 6
6.1. Application Method .........................................................................................................................6
6.2. Attention for Operation................................................................................................................... 9
6.3. Clinical Restrictions...................................................................................................................... 10
7. Maintenance, Transportation and Storage.......................................................................................10
7.1. Cleaning and Disinfection............................................................................................................. 10
7.2. Maintenance..................................................................................................................................10
7.3. Transportation and Storage ...........................................................................................................10
8. Troubleshooting.................................................................................................................................. 11
9. Key of symbols....................................................................................................................................12
10. Function specification......................................................................................................................13
Appendix 1............................................................................................................................................... 14
TORONTEK-E400 Pulse Oximeter

1. Safety
1.1. Instructions for Safe Operations
Inspect the main unit and all accessories periodically to ensure that there is no visible damage
that may affect the patient’s safety, and monitor the performance of cables and transducers. It is
recommended that the device should be inspected at least once a week. If there is obvious
damage, stop using the device.
Necessary maintenance must be performed by qualified service engineers ONLY. There are no
user serviceable parts and users are not permitted to service the device by themselves.
The Oximeter must not be used together with accessories not specified in the User Manual.
Only accessories specified or recommended by the manufacturer can be used with this device.
This product is calibrated before leaving the factory.
1.2. Warning
Explosive hazard—DO NOT use the Oximeter in an environment with flammable gas, such as
some ignitable anesthetic agents.
DO NOT use the Oximeter while the patient is measured by MRI and CT.
Be careful with the use of the wrist strap. Improper use of the wrist strap will cause damage not
covered under the manufacturer’s warranty. Swinging the device by the wrist strap will void the
warranty. Please do not use the wrist strap if allergic to the material.
The disposal of this instrument and its accessories and packaging (including batteries, plastic
bags, foams and paper boxes) should follow local laws and regulations.
Please check the packaging before use to make sure the device and accessories are in complete
accordance with the packaging list or else the device may not work normally.
Use only accessories approved by the manufacturer or else risk damaging the device.
Please only use the battery charger which is accompanies by the product and is in compliance
with the requirements of IEC 60601-1. Using a different charges might damage the device.
Do not use the device while it is charging.
1.3. Hazards
Keep the Oximeter away from dust, vibrations, corrosive substances, explosive materials,
high temperatures and moisture.
Stop operating the Oximeter if it gets wet.
DO NOT use the device immediately after moving it from a cold environment to a warm or
humid environment.
DO NOT operate the keys on the front panel with sharp objects.
High temperature and high pressure steam disinfection of the Oximeter is not permitted.R
efer to the User Manual in the relative chapter (7.1) for instructions on cleaning and
disinfection.
1
TORONTEK-E400 Pulse Oximeter

DO NOT immerse the Oximeter in liquid. If it needs cleaning, wipe the surface with medical
alcohol using a soft material. Do not spray any liquid directly onto the device.
When cleaning the device with water, ensure that the temperature of the water is lower than
60℃.
Thin or cold fingers will affect the normal measure of the patient's SpO2and pulse rate. Be
sure to clip a thick finger such as the thumb or middle finger deeply enough into the probe.
The update period of data is less than 5 seconds. This is changeable according to different
individual pulse rates.
The waveform is normalized. Please read the measured value when the waveform on-screen
is uniform and steady-going. This measured value is the optimal value and this waveform is
the standard one.
If abnormal conditions appear on the screen during the test process, pull out the finger and
reinsert it to restore normal use.
The device has a lifespan of three years after the first electrified use.
The wrist strap attached to the device is made from non-allergenic material. If you are
sensitive to the wrist strap, stop using it. In addition, pay attention to the use of the wrist
strap. To avoid harm, DO NOT wear it around the neck.
This device has an alarm function.
Provided the alarm function is turned on, an alarm will automatically go off if the measured
data goes beyond the highest or lowest limit.
The alarm function can either be paused or turned off (default setting). The function can be
turned back on through menu operation. Please refer to Chapter 6.1 for more information on
the alarm function.
The device may not work for all patients. If you are unable to achieve stable readings,
discontinue use.
A flexible circuit connects the two parts of the device. DO NOT twist or pull on the
connection.
2. Overview
The peripheral capillary oxygen saturation (SpO2) is the percentage of oxyhemoglobin (HbO2) in the
total hemoglobin (Hb) in the blood, or the oxygen concentration in the blood. It is an important bio-
parameter for respiration. A number of diseases relating to the respiratory system may cause a decrease
of SpO2in the blood. Furthermore, some other causes such as the malfunction of the body's self-
adjustment, damage during surgery, and injuries caused by some medical examinations could also lead to
a low oxygen supply in the human body. As a consequence, symptoms such as vertigo, impotence and
vomiting would appear. Serious symptoms might endanger the patient’s life. Therefore, prompt
information of patients' SpO2is of great help for doctors to discover potential dangers and is of great
importance in the clinical medical field.
The Pulse Oximeter features a small size, low power consumption, convenient operation and portability.
The patient only needs to put one of their fingers into a probe for diagnosis and a display screen will
directly show the measured value of pulse oxygen saturation with high accuracy.
2.1. Features
A. Operation of the product is simple and convenient.
B. The product is small in size and light in weight, making it convenient to carry.
C. The device has low power consumption.
2
TORONTEK-E400 Pulse Oximeter
2.2. MajorApplications and Scope of Application
The Pulse Oximeter can be used to measure the pulse oxygen saturation and pulse rate through a finger.
The device is suitable for use in the home, hospitals, oxygen bar, community health care, and physical
care in sports (it can be used before or after doing sports and is not recommended to be used during
sporting activities).
The problem of overrating will emerge if the patient is suffering from toxicosis caused by
carbon monoxide. The device is not recommended to be used under these circumstances.
2.3. Environment requirements
Storage Environment
a) Temperature :-40℃~+60℃
b) Relative humidity :≤95%
c) Atmospheric pressure :500hPa~1060hPa
Operating Environment
a) Temperature:10℃~40℃
b) Relative Humidity :≤75%.
c) Atmospheric pressure:700hPa~1060hPa
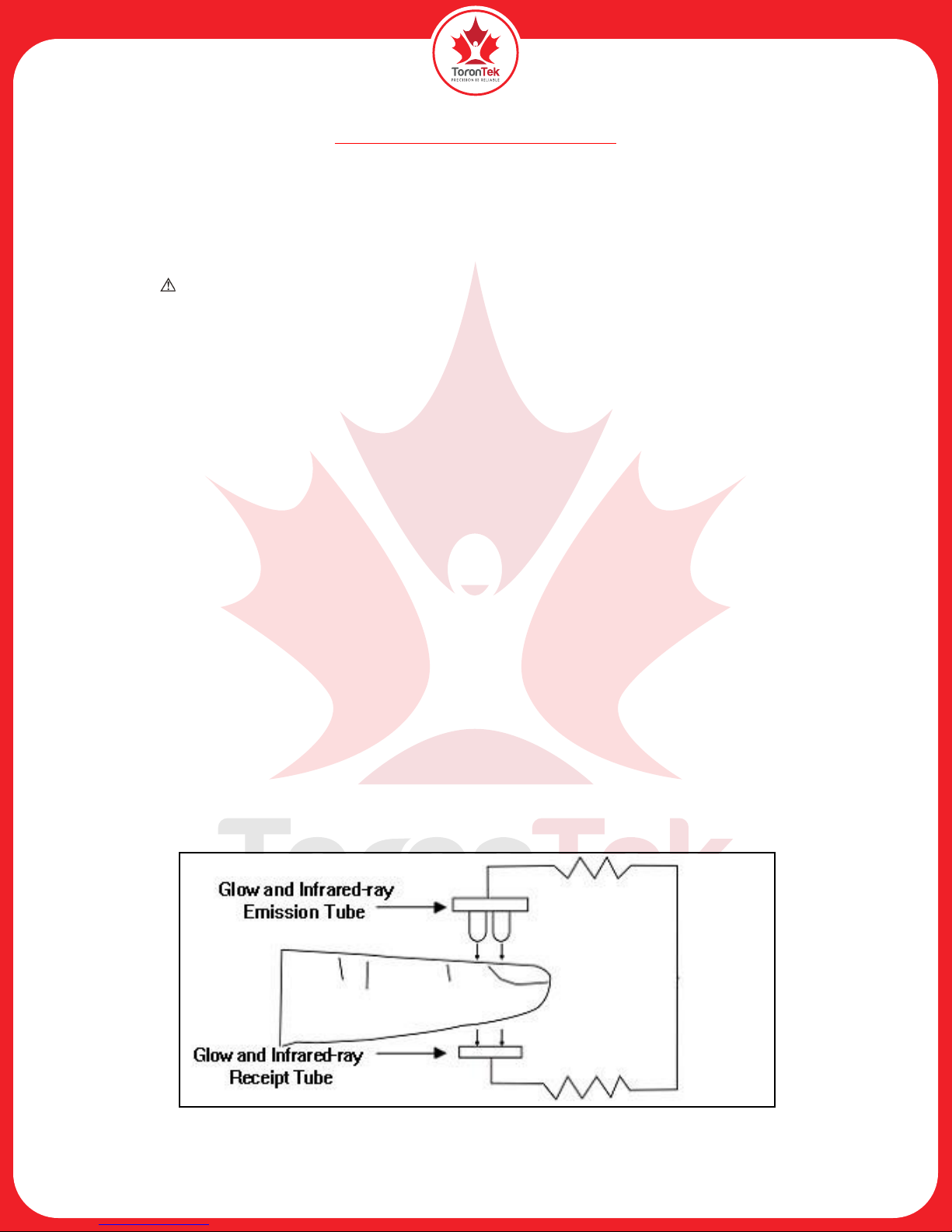
3. Principle
Operation principle of the Oximeter: Photoelectric Oxyhemoglobin Inspection Technology is applied
together with Capacity Pulse Scanning & Recording Technology so that two beams of light of different
wavelengths can be focused onto a human nail tip through a perspective clamp finger sensor. A measured
signal is obtained by a photosensitive element and acquired information will be shown on-screen through
treatment in electronic circuits and microprocessors. calculation of blood oxygen level the device uses a
formula to process the data according to Lambert Beer Law and Spectrum Absorption Characteristics of
Reductive Hemoglobin (Hb) and Oxyhemoglobin (HbO2) in glow & near-infrared zones.
Figure 1
3
TORONTEK-E400 Pulse Oximeter
Table of contents
Other ToronTek Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual