4 of 20
MA 01347, Issue 1, November 2015
1.0 i-digits™quantum
1.1 Product Description
design. dexterity. intelligent motion.
Based on the industry-leading design of our i-limb™ product
range, i-digits™ quantum combines unsurpassed functionality
with style. i-digits™ quantum incorporates gesture control via
the patented and ground-breaking i-mo™ technology and is
the first partial hand prosthesis that can change grips with a
simple gesture.
Key features include:
•Smarter
- gesture control powered by i-mo™ technology uses
simple gestures to change grips
- proximity control made available via grip chips™
technology
•Faster
- adjustable speed boost increases speed up to 30%
•Stronger
- up to 30% more power when needed
- improved component design for easier and more reli-
able fabrication
- 50% longer battery life
•Smaller
- new form fitting design decreases device size in
every dimension
- smaller digits now available
We are pleased that you and your clinical team decided that
i-digits™ quantum is the most appropriate prosthesis for your
needs. You will have discussed your functional goals with your
clinical team. This manual, along with the training and sup-
port of your clinical team, should help you understand how
i-digits™ quantum will help you accomplish these goals.
1.2 Intended Use
i-digits™ quantum is intended to be used by individuals with
partial hand loss or deficiency. Devices are suited to individu-
alswith any 3, 4 or 5 digit loss, while patients or users with 1 or
2 digit loss are also indicated when the digits of loss are either
the thumb or the main digits of opposition, namely the index
and middle fingers.
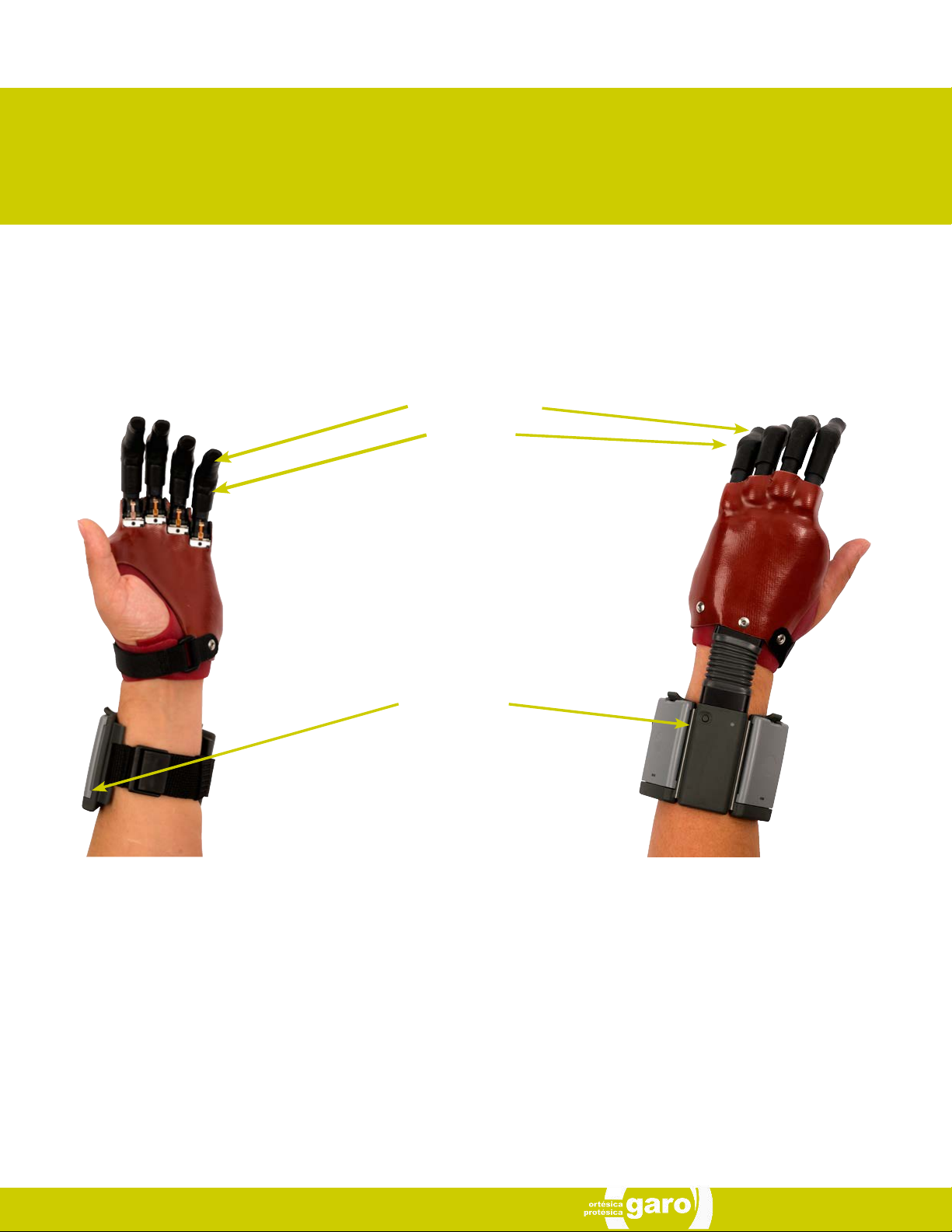
i-digits™ quantum is a fully customized partial hand prosthesis
with individually powered fingers. i-digits™ quantum fingers
move independently and bend at the joint to work in conjunc-
tion with any remaining fingers to help significantly increase
functional capabilities.
Each digit of the device has its own motor that allows the digits
to run until they meet the object being grasped. The result of
this is the device taking the shape of that object (compliant
grip). The power for the prosthesis comes from two batteries
which are located in the wristband. Initially, you and your clini-
cal team will develop control of opening and closing your hand.
At this stage, you should be able to carry out a wide range of
functional daily activities using your i-digits™ quantum device.
It takes time and practice to gain control and master the best
way to perform tasks with your prosthetic device. The pace at
which individuals gain this control varies.
Your i-digits™ quantum is covered under the Touch Care pro-
gram. Please contact your clinician for details about your spe-
cific Touch Care coverage.