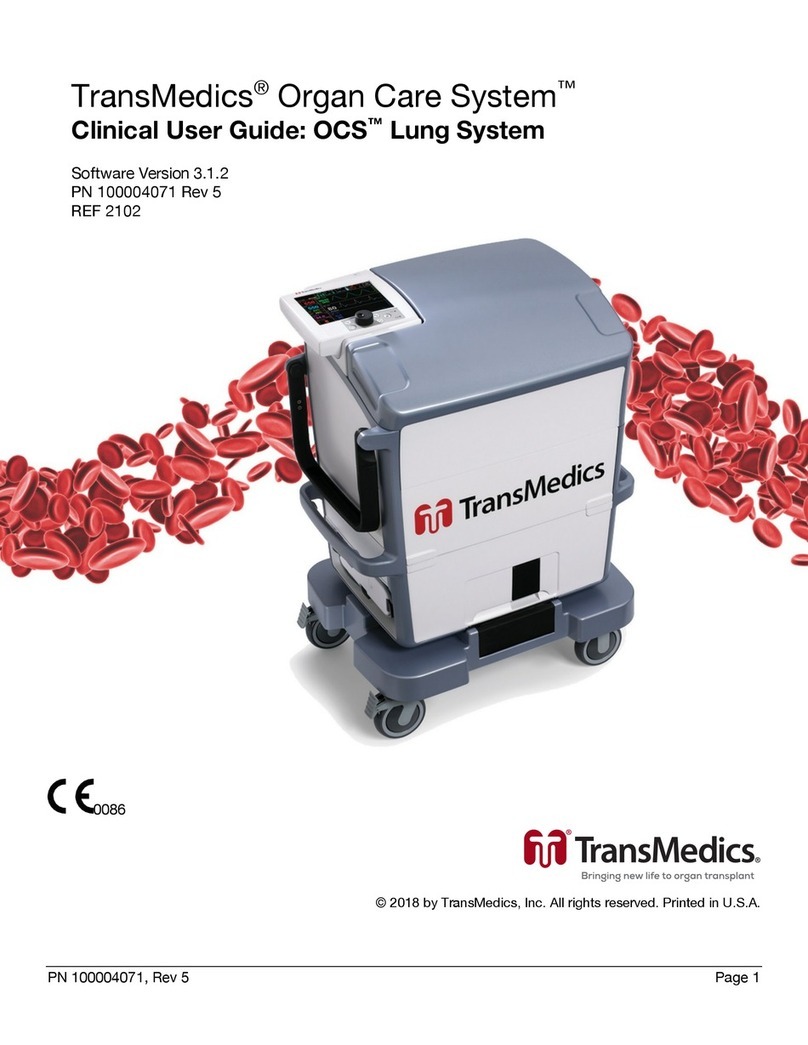
TransMedics Organ Care System Manual

PN 100004070, REV 6 Page 1
TransMedics® Organ Care System™
Technical User Guide: OCSTM Lung System
Software Version 3.1.2
PN 100004070 Rev 6
REF 2103
2797
© 2019 by TransMedics, Inc. All rights reserved. Printed in U.S.A.

PN 100004070, REV 6 Page 2
Manufacturer’s Address:
TransMedics, Inc.
200 Minuteman Rd., Suite 302 Andover, MA 01810, USA
Tel: +1 978 552 0999
Fax: +1 978 552 0978
Website: www.transmedics.com
2797
This device complies with the Medical Device Directive 93/42 EEC.
Authorized EU Representative:
Healthlink Europe BV De Tweeling 20-22
5215 MC’s Hertogenbosch, The Netherlands
Tel: +31-(0)13-5479316
Patents:
U.S. Patents 6,046,046, 6,100,082; International Patents EU, UK, FR, ES, IT, BE, DK, FI, IE, LU, MC, NL, PT, CH, SE 1017274, DE
69819759.3-08, AU728233, ATE253819; Additional Patents Pending.
Manual PN & Rev
PN 100004070 REV 6
CAUTION: United States federal law restricts this device to sale by or on the order of a physician.
This document and the information contained in it is proprietary and confidential information of TransMedics and may not be
reproduced, copied in whole or in part, adapted, modified, disclosed to others, or disseminated without the prior written permission
of the TransMedics Legal Department. This document is intended to be used by customers and is licensed to them as part of their
TransMedics equipment purchase. Use of this document by unauthorized persons is strictly prohibited.
TransMedics provides this document without warranty of any kind, implied or expressed, including, but not limited to, the implied
warranties of merchantability and fitness for a particular purpose.
TransMedics has taken care to ensure the accuracy of this document. However, TransMedics assumes no liability for errors or
omissions and reserves the right to make changes without further notice to any products herein to improve reliability, function, or
design. TransMedics may make improvements or changes in the products or programs described in this document at any time.
This product may contain remanufactured parts equivalent to new in performance, or parts that have had incidental use.
TRANSMEDICS®, OCS™, and the TransMedics logo are trademarks of TransMedics, Inc., Andover, MA, USA. All rights reserved. Non-
TransMedics product names may be trademarks of their respective owners.
©2019 TransMedics, Inc. All rights reserved.

Table of Contents
PN 100004070, REV 6 Page 3
Table of Contents
GLOSSARY OF TERMS .........................................................................................................................6
1. CHAPTER 1: READ THIS FIRST ....................................................................................................9
1.1. Directions to User .................................................................................................................... 9
1.2. User Training Requirements .................................................................................................... 9
1.3. Indications for Use ................................................................................................................... 9
1.4. Conventions ............................................................................................................................. 9
1.5. Upgrades and Updates........................................................................................................... 11
1.6. Supplies .................................................................................................................................. 11
1.7. Contacting TransMedics......................................................................................................... 11
2. CHAPTER 2: SAFETY INFORMATION.........................................................................................12
2.1. Electrical Safety...................................................................................................................... 12
2.2. Mechanical and System Safety .............................................................................................. 13
2.3. Operator Safety...................................................................................................................... 13
2.4. Patient and Organ Safety ....................................................................................................... 15
2.5. Component Safety.................................................................................................................. 15
2.6. Symbols Used on the System ................................................................................................. 15
2.7. Using Aseptic Technique ........................................................................................................ 17
2.8. Moving the System................................................................................................................. 17
2.9. Storing the System Between Uses ......................................................................................... 18
2.10. Shipping, Handling and Storage Requirements ..................................................................... 19
2.11. Periodic Safety Tests .............................................................................................................. 19
2.12. Explosion Hazard.................................................................................................................... 20
2.13. Electromagnetic Compatibility............................................................................................... 20
2.14. Electromagnetic Interference (EMI)....................................................................................... 20
2.15. Handling Batteries.................................................................................................................. 20
2.16. Handling Pressurized Gas Cylinders ....................................................................................... 21
2.17. Disposal of the System and Components .............................................................................. 22
3. CHAPTER 3: OVERVIEW...........................................................................................................23
3.1. System Components .............................................................................................................. 23
3.2. OCS™ Lung System................................................................................................................. 24
3.3. Lung Perfusion Set Components............................................................................................ 29
3.4. Overview of Use ..................................................................................................................... 35
4. CHAPTER 4: SYSTEM SETUP AND CONNECTIONS......................................................................39
4.1. Connecting the System to AC Power ..................................................................................... 39
4.2. Checking Battery Power......................................................................................................... 40
4.3. Docking and Undocking the Wireless Monitor ...................................................................... 42
4.4. Using the Lung Preservation Gas Cylinders............................................................................ 44
4.5. Using the Lung Monitoring Gas Cylinder ............................................................................... 48
4.6. Returning an EMPTY Gas Cylinder to TransMedics................................................................ 50
4.7. Replacing a Yoke Gasket ........................................................................................................ 51
4.8. Using the TransMedics Data Cards ........................................................................................ 51
4.9. Using the Mobile Base ........................................................................................................... 52
4.10. Lung Perfusion Module Preparation ...................................................................................... 53

Table of Contents
PN 100004070, REV 6 Page 4
5. CHAPTER 5: WIRELESS MONITOR OVERVIEW ..........................................................................54
5.1. Wireless Monitor Components.............................................................................................. 54
5.2. Wireless Monitor Controls ..................................................................................................... 55
5.3. Wireless Monitor Display Overview....................................................................................... 56
6. CHAPTER 6: MANAGING THE SYSTEM .....................................................................................61
6.1. Managing Sessions ................................................................................................................. 61
6.2. Using the Configuration and Action Menus........................................................................... 63
6.3. Configuring Session Settings .................................................................................................. 65
6.4. Configuring System Settings .................................................................................................. 70
6.5. Managing Default Configuration Settings.............................................................................. 71
6.6. Adjusting the Pump................................................................................................................ 74
6.7. Managing Alarms ................................................................................................................... 75
6.8. Managing Real-Time Waveforms........................................................................................... 78
6.9. Managing Historical Trend Graphs......................................................................................... 80
6.10. Starting and Resetting the Perfusion Clock............................................................................ 82
6.11. Managing Blood and Perfusate Sample Data ........................................................................ 82
6.12. Using Annotations.................................................................................................................. 85
6.13. Calibrating the SaO2/HCT Probe............................................................................................. 87
6.14. Displaying System Status........................................................................................................ 88
7. CHAPTER 7: CLEANING AND MAINTAINING THE OCS™.............................................................89
7.1. Routine Inspection Before and After Use .............................................................................. 89
7.2. Infection Control .................................................................................................................... 90
7.3. Handling Blood-Contaminated Components ......................................................................... 90
7.4. Cleaning and Disinfecting the System after Use .................................................................... 90
7.5. Cleaning and Disinfecting the Probes .................................................................................... 96
7.6. Cleaning and Maintenance Task Checklist............................................................................. 97
8. CHAPTER 8: SYSTEM SPECIFICATIONS......................................................................................99
8.1. Safety & Regulatory Specifications ........................................................................................ 99
8.2. Electrical and Physical Specifications ..................................................................................... 99
8.3. Electromagnetic Emissions................................................................................................... 100
8.4. Essential Performance ......................................................................................................... 103
8.5. Accuracy of Displayed Values............................................................................................... 103
9. CHAPTER 9: TROUBLESHOOTING...........................................................................................104
9.1. Emergency Support.............................................................................................................. 104
9.2. Technical Service Follow-Up ................................................................................................ 104
9.3. Troubleshooting the OCS™ .................................................................................................. 104
9.4. Standby-Cycling the System................................................................................................. 104
9.5. Power-Cycling the System.................................................................................................... 105
9.6. Unlatching and Latching the Lung Perfusion Module.......................................................... 105
9.7. Early Termination Procedure ............................................................................................... 105
9.8. Resetting the System ........................................................................................................... 117
9.9. Wireless Monitor Failures .................................................................................................... 117
9.10. Shipping Equipment for Service ........................................................................................... 118
9.11. Aligning the Bellows Plate.................................................................................................... 118
9.12. Bellows Alignment Procedure.............................................................................................. 118

Table of Contents
PN 100004070, REV 6 Page 5
9.13. Known Limitations of the OCS™ 3.1.2-C Software............................................................... 119
10. PARTS AND SUPPLIES ...........................................................................................................120
10.1. Contacting TransMedics....................................................................................................... 120
10.2.Ordering Parts and Supplies................................................................................................. 120
APPENDIX A. SYMBOL GLOSSARY .............................................................................................124

Glossary of Terms
PN 100004070, REV 6 Page 6
GLOSSARY OF TERMS
Term Meaning
ABG Arterial Blood Gas
Annotations Notes or comments entered through the Wireless Monitor during the session that are automatically
stamped with the time of entry and saved in the session file.
BPM Breaths/minute
Bronchoscope
Mode
Ventilation of the lungs with ambient air, to allow examination of the interior of the lungs with a
Bronchoscope.
Bronchoscope port Port on the Lung Perfusion Module through which a Bronchoscope probe may be inserted to inspect the
interior of the lung.
Circuit Refers to the perfusate loop in the Lung Perfusion Module.
Continuous
Monitoring Mode
The Ventilator Mode in which the OCS™ Lung System continuously deoxygenates the perfusate by supplying
Lung Monitoring Gas into the gas exchanger. At the same time, ambient air is used to ventilate the lung.
Medical professionals may evaluate the capabilities of the lungs according to their clinical judgment by
comparing the base O2saturation of the deoxygenated perfusate to the O2saturation of the perfusate
exiting the lung.
Cuvette An adapter on the Lung Perfusion Module used for an oxygen saturation measurement probe.
Data Card A removable SD Data card used to store perfusion, ventilation, and monitoring parameters from the current
session, which can be downloaded and analyzed on a personal computer.
Expiratory Time The time during the respiration cycle between the end of the plateau time and the beginning of the next
inspiration. This includes the time that the lungs are exhaling, and the rest time until the start of the next
inspiration.
FiO2 Fraction of inspired oxygen.
Gas Intake Port Ventilation inlet port on the Lung Perfusion Module through which room air enters the ventilation circuit
during Monitoring Modes.
HCT% Hematocrit, expressed as a percentage by volume.
IE Ratio Inspiration / Expiration Time Ratio
Inspiratory Time The time during the respiration cycle when gas flows into the lungs.
LA Left Atrial
LPM Lung Perfusion Module
LPS Lung Perfusion Set
L/min Liters/minute
MDI Port Metered Dose Inhaler port on the Lung Perfusion Module through which MDI drugs may be injected into
the lungs
mL/hr Milliliters per hour
mL/min Milliliters per minute
mmHg Millimeters of mercury.

Glossary of Terms
PN 100004070, REV 6 Page 7
Term Meaning
Mobile Base The removable Mobile Base has four wheels, with brakes on the front wheels. The Mobile Base can be
installed as needed during system use. During transport, raise the two-position handle to push the system.
With the Mobile Base removed, you can set the system flat or carry it with the lift handles.
Organ Care System The Organ Care System (OCS™) houses the removable Wireless Monitor, circulatory pump driver, multi-
mode Ventilator drive and control, batteries, data card, gas delivery subsystem, and reusable flow and
pulse oximeter probes. When in active use, it houses the disposable Lung Perfusion Module.
PA Pulmonary artery
PaO2Partial pressure of oxygen in mmHg in arterial (oxygenated) perfusate.
PAP Pulmonary Artery Pressure. The perfusate pressure in mmHg at the Pulmonary Artery going into the lungs.
Pause Preservation
Mode
A Ventilator mode in which the bellows remain stationary and the OCS™ Lung System achieves a static level
of lung inflation. Pause Preservation enables oxygenation of perfusate prior to lung instrumentation using
the Lung Preservation Gas.
PAWP Peak Air Way Pressure. The peak pressure in the lungs at the end of the inspiration. When the measured
PAWP reaches the user-set PAWP limit, the Ventilator will stop. PAWP corresponds to Peak Inspiratory
Pressure on mechanical Ventilators.
PEEP Positive End Expiratory Pressure. The pressure maintained in the lungs by the Ventilator at the end of the
expiration phase.
Perfusate The fluid pumped through the lung that delivers dissolved gases and nutrients
Plateau Time The time between the end of inspiration and the start of expiration.
Power-cycle To Power-cycle the system, use the On/Off switch on the side of the OCS™ Lung Console to turn the system
OFF, wait 10 seconds, and then turn it ON.
Preservation Mode A Ventilator mode in which the OCS™ Lung System operates with the lung rebreathing the same captive
breath. A small percentage of fresh Lung Preservation Gas is injected into the ventilation circuit to maintain
the required gas concentration and to maintain Positive End Expiratory Pressure (PEEP).
Priming Inlet Port Port on the Lung Perfusion Module through which priming solution and other large-volume perfusate
components flow into the reservoir.
Priming Solution The sterile OCSTM Lung solution added to the reservoir through the priming inlet port to preserve the lung
and to supplement the volume of other perfusate components. Priming solution is circulated through the
Lung Perfusion Module circuit along with other perfusate components prior to organ connection and during
organ preservation
Pump Compliance
Chamber
Located between the circulatory pump and the perfusate warmer, the red-colored pump flow compliance
chamber smooths out the pulsatile flow from the pump.
Pump Flow Probe A probe that you attach to the Lung Perfusion Module. It is used to measure OCS™ pump flow.
PvO2 Partial pressure of oxygen gas in mmHg in venous (deoxygenated) perfusate.
Relief Port Port on the Lung Perfusion Module through which lung exhalation gas is output.
RR Respiration Rate. Number of respiration cycles per minute in units of breaths/minute.
RESP Name of the airway pressure/respiration pressure, waveform displayed on the Wireless Monitor
Run Mode Power mode where the system is on, the Wireless Monitor is active, and the pump and Ventilator can
operate.

Glossary of Terms
PN 100004070, REV 6 Page 8
Term Meaning
SaO2 Oxygen saturation of arterial (oxygenated) perfusate, expressed as a percentage and measured at the
output of the lung at the LA drain.
SaO2Hematocrit
Probe
An OCS™ Lung Console probe that you attach to the Lung Perfusion Module. It is used to measure the
arterial oxygen saturation and the hematocrit of the perfusate leaving the lung through the LA.
Session A session is created in internal system memory when the system is set to Run Mode. Every time Run Mode
is entered, you can choose whether to continue using the last session file or create a new one. In ordinary
circumstances, data from all procedures associated with an organ should be documented in only one
session. The system logs all system error events, all alarm events, trend data for each parameter at 2-
minute intervals, and all system operating events that occur in each session.
Standby-cycle To Standby-cycle the system, press the Standby button to switch from Run Mode to Standby Mode and
then back to Run Mode. The system will automatically run the Self Test.
Standby Mode A power mode where the system is on but the Wireless Monitor is off and no ventilation or perfusion may
be performed. Standby Mode is the mode used during OCS™ storage; organs cannot be preserved in this
mode. The OCS™ must be plugged in to AC power to avoid battery depletion when storing the OCS™ in this
mode.
SvO2Oxygen saturation of venous (deoxygenated) perfusate, expressed as a percentage and measured at the
input to the lung on the PA line.
SvO2Hematocrit
Probe
An OCS™ Lung Console probe that you attach to the Lung Perfusion Module. It is used to measure the
venous oxygen saturation and hematocrit of the perfusate entering the lung through the pulmonary artery
cannula.
Temp Temperature of perfusate supplied to the lung, displayed on the Wireless Monitor in degrees Celsius.
Trend A trend contains the most recent 24 hours of data, updated every two minutes. Each data point represents
the average value calculated over the previous two minutes. You can configure trend data to display on the
middle and bottom frames on the Wireless Monitor.
TV Tidal Volume. The volume of air breathed in and out of the lungs during a respiration cycle.
Update Bar A vertical line displayed on the waveform. Newest data is displayed to the immediate left of the update bar.
The bar is aligned with other update bars displayed at the same time.
Ventilator Lines
connector
Integrated pneumatic connector that conveys ventilation and control gas between the OCS™ Lung Console
and the Lung Perfusion Module.
VR Vascular Resistance. This is a measure of the resistance to flow that must be overcome to push perfusate
through the vasculature of the lungs. It is calculated as (80* mean PAP)/(Pump Flow) and displayed in units
of (dyne*sec)/cm5.
Waveform Real-time waveforms display continuously updated data. The waveforms are drawn from left to right with
the most current data. An update bar displays the oldest data first. If more than one graphic frame is
configured to show real-time waveforms, the update bars are automatically synchronized. The airway
pressure waveform is always displayed in the top-most frame on the Wireless Monitor. Use the
Configuration Menu to configure which waveforms are displayed in the middle and bottom frames on the
Wireless Monitor.
Wireless Monitor A small, dockable monitoring system with an LCD screen and controls for configuring system functions and
screen displays, and for adjusting system settings during preservation. When removed from its docking
station on the OCS™ Lung Console, the Monitor operates wirelessly, powered by its own battery.

Chapter 1: Read This First
PN 100004070, REV 6 Page 9
1. CHAPTER 1: READ THIS FIRST
This chapter contains important information about the documentation for your TransMedics® Organ Care
System (OCS™) Lung System and about contacting TransMedics.
1.1. Directions to User
This manual provides detailed instructions about using the OCS™ Lung System, including an overview of the
system, how to set up the system, understanding the Wireless Monitor and its controls and functions,
troubleshooting, and cleaning and maintaining the system. For step-by-step instructions for using the system
before, during, and after organ collection, preservation, and transport, as well as a summary of the clinical
results, see the TransMedics Clinical User Guide: OCS™ Lung System. Both guides are to be reviewed prior to
using the system, noting the Warnings and Cautions throughout the guides.
The OCS™ Lung System can only be purchased upon order of a physician. A TransMedics representative must
install and activate each newly purchased system before a qualified health care professional can use it.
1.2. User Training Requirements
The OCS™ Lung System enables medical professionals to monitor key parameters that may be useful in
assessing organ condition and function according to their clinical judgment. The system is intended for use
only by qualified healthcare professionals specializing in lung transplants and trained in the use of the OCS™
Lung System.
Completion of the TransMedics training program is required for every new lung transplant center prior to
starting an OCS™ Lung System program at their institution. All team members at an institution must be
trained. The training consists of initial hands-on training and periodic refresher training as needed.
1.3. Indications for Use
The indications for use are as follows:
The TransMedics Organ Care System (OCS) Lung is a portable normothermic organ perfusion,
ventilation and monitoring medical device indicated for preservation of standard criteria donor lung
pairs and for preservation of donor lung pairs initially deemed unacceptable for procurement and
transplantation based on the limitations of cold static preservation. The device allows for ex vivo
assessment of donor lungs prior to transplantation.
For contraindications, warnings, and cautions specific to clinical use, as well as a summary of the INSPIRE
Study and EXPAND Study clinical results, refer to the TransMedics Clinical User Guide: OCS™ Lung System.
1.4. Conventions
The terms OCS™ Lung System, OCS™, and system are used interchangeably throughout this manual to refer to
the TransMedics OCS™ Lung System. The system uses consistent conventions throughout the interface and
accompanying documentation to make it easy for you to learn and use. The documentation follows these
conventions:

Chapter 1: Read This First
PN 100004070, REV 6 Page 10
•All step-by-step procedures are numbered, and all sub-procedures are lettered. You must complete
steps in the sequence they are presented to ensure success.
•Bulleted lists indicate general information about a particular function or procedure. They do not
imply a sequential procedure.
•The term Press means to press the push button or the rotary knob on the Wireless Monitor.
•Highlight means to turn the rotary knob to move a highlight to a screen element.
•Select and Enter means to highlight the selection and press the rotary knob on the Wireless
Monitor to confirm selection of a highlighted item.
•Controls, labels, and selectable menu items are shown in bold type and are capitalized exactly as
they appear. For example, Return, Cancel.
•The terms Standby Mode, Preservation Mode, and Monitoring Mode are capitalized so you can
easily determine the mode.
•The terms Donor, Donor Site, Recipient, and Recipient Site are capitalized.
•The term circuit refers to the Lung Perfusion Module with the perfusate running through it.
•Warnings, Cautions, and Notes are set apart from other text. See below for definitions.
•The left side of the system is to your left as you stand in front of the system, facing the system. The
front of the system is nearest you with the push handle on the left side.
•The Glossary lists terms used throughout this manual and their definitions.
Warnings, Cautions, and Notes
The Warnings, Cautions, and Notes in this user manual are specific to the controls and functions of the OCS™
Lung System.
WARNING—A Warning alerts you to a potential serious outcome, adverse event or safety hazard. Failure to
observe a warning may result in loss of organ, death, or serious injury.
CAUTION—A Caution alerts you to situations where special care is necessary for the safe and effective use of
the product. Failure to observe a caution may result in minor or moderate personal injury or damage to the
product or other property, and possibly a risk of more serious injury.
NOTE—A Note brings your attention to important information that will help you operate the system more
effectively.

Chapter 1: Read This First
PN 100004070, REV 6 Page 11
1.5. Upgrades and Updates
TransMedics is committed to innovation and continued improvement. Upgrades may be announced that
consist of hardware or software improvements. Updated documentation will accompany those system
upgrades.
1.6. Supplies
The components, accessories, and supplies required when using the OCS™ Lung System must be used in
accordance with this user manual, associated documents, and accepted medical standards.
CAUTION—Only accessories and supplies purchased from or recommended by TransMedics, Inc. are to be used with the OCS™
Lung System. Use of accessories and supplies other than those supplied by or recommended by TransMedics may cause system
malfunction and invalidate the TransMedics warranty.
For details on what is included with your OCS™ Lung System and Lung Perfusion Set, see Chapter 3: Overview.
To order additional parts and supplies, see Section 10. Other materials, not supplied by TransMedics, are
required to operate the OCS™. See the TransMedics Clinical User Guide: OCS™ Lung System for details.
1.7. Contacting TransMedics
Customer service representatives are available worldwide to answer questions and to provide maintenance
and service. Please contact TransMedics for assistance at +1 978-552-0999.
You can also contact one of the following offices for referral to a customer service representative, or visit the
TransMedics website: www.transmedics.com.
Corporate and North American Headquarters
TransMedics, Inc.
200 Minuteman Road, Suite 302
Andover, MA 01810, USA
Tel: +1 978-552-0999
Fax: +1 978-552-0978
Authorized EU Representative
Healthlink Europe BV
De Tweeling 20-22
5215 MC’s Hertogenbosch
The Netherlands
Telephone: +31(0) 13 547 9316

Chapter 2: Safety Information
PN 100004070, REV 6 Page 12
2. CHAPTER 2: SAFETY INFORMATION
The TransMedics® OCS™ Lung System can be purchased only upon order of a physician and is intended for use
by qualified medical personnel who have received TransMedics training. Only a trained operator can use the
system.
This chapter provides information about safety issues that may arise. Read this section before you use the
OCS™ Lung System or any of its components. Be sure to read all applicable usage, patient-safety, operator-
safety, and electrical safety guidelines in this manual.
If you have any comments or questions about safety, contact your TransMedics representative. For
precautions for handling and disposing of blood-contaminated equipment and materials, see Chapter 7:
Cleaning and Maintaining the OCS™.
2.1. Electrical Safety
This section provides warnings and cautions related to electrical safety.
WARNINGS—
No modification of this equipment is allowed.
Failure to abide by the precautions detailed in this section may cause the system and its use to be out of compliance with
regulations and places personnel and any people near the system at risk of injury or death.
Connect the system AC power cord only to a properly grounded 100V to 240V, 50/ 60 Hz Hospital Grade AC outlet.
To avoid risk of electric shock, this equipment must only be connected to a mains supply with protective earth.
If you have any doubt about the integrity or suitability of the external power or of the cable, plug, or connector, do not connect the
power cord. To avoid potential electrical hazards, allow the system to function on OCS™ battery power only, until appropriate
external power is available or any problems have been resolved.
Never use a converter adapter to plug the three-pronged AC plug into a two-pronged ungrounded wall outlet. Doing so may result in
electric shock to the operator and damage to the equipment.
To avoid electrical shock, use only the power cords supplied by TransMedics for the OCS™, and connect only to properly grounded
wall outlets.
Do not use additional cables or extension cords with the TransMedics system.
Do not remove any system covers except those necessary to access the system for use, as described elsewhere in this manual. Any
other covers are to be removed by qualified TransMedics service personnel only. Accidentally contacting the electrical circuits inside
the housings may result in electric shock to the operator and damage to the equipment.
Internal fuses are not field serviceable.
Before cleaning or servicing the system, disconnect all external power sources.
To completely disconnect AC power, you must unplug the system from the AC power receptacle and turn off the power switch.
Neither the button nor the On/Off switch completely disconnects power.

Chapter 2: Safety Information
PN 100004070, REV 6 Page 13
To fully de-power the system, you must unplug the system from the AC power receptacle and either fully deplete the OCS™
batteries, or remove them completely from the system.
CAUTION—Use the system only at the temperatures, relative humidities, and altitudes specified in Table 8-2, “OCS™: Electrical
and Physical Specifications.”
2.2. Mechanical and System Safety
Shipment Inspection. Inspect TransMedics packaging to ensure there has been no shipping damage.
Periodic Visual Inspection. Before and after each use, inspect the system for any physical damage that might
require service or replacement of an individual component in time for the next use.
Regular Cleaning and Disinfection. Keep surfaces and cables clean, cleaning all surfaces and cables before and
after each use. Clean and disinfect any bodily fluid or blood-contaminated areas of non-sterile parts of the
system immediately after removing and properly disposing of the Lung Perfusion Module. Clean and disinfect
only as described in Chapter 7: Cleaning and Maintaining the OCS™.
Preventative Maintenance. The OCS™ Lung Console should be tested by a qualified TransMedics
representative at least once per year. At this time, the following components should be serviced: ventilation
filters. The use life of the OCS™ Lung Console is expected to be at least five years with a rate of use of 50
preservation sessions per year.
WARNINGS—Do not immerse an OCS™ battery in water, and do not allow liquids to enter the slot or the electrical contacts at the
back of the battery during cleaning. Lithium may react violently when mixed with water, leading to possible battery leakage, smoke,
and fire.
CAUTIONS—
Use only accessories and supplies purchased from or recommended by TransMedics. Use of accessories and supplies other than
those supplied by or recommended by TransMedics may cause organ damage and will invalidate the TransMedics warranty. (This
manual details approved accessories and supplies as relevant to system operation.)
Do not attempt to sterilize the OCS™ or any of its non-sterile components. Doing so may damage the system.
Do not use any cleaning or disinfection agents other than those prescribed in this manual.
Doing so may lead to component damage, or interference with proper system operation.
2.3. Operator Safety
Intended Users: The OCS™ is intended for use only by qualified health care professionals specializing in organ
transplants and trained in use of the OCS™.
Before Using the System: Each user should carefully review the manual, noting especially the Warnings and
Cautions throughout and the information in this chapter.

Chapter 2: Safety Information
PN 100004070, REV 6 Page 14
Careful Cable Routing: Carefully route system power cables and other cables to reduce the possibility of
tripping or disrupting operation during system use or transport.
Disposal of Blood-Contaminated Materials: Always follow your institutional protocols for handling and
disposal of blood-contaminated materials.
Not Serviceable by User: There are no user-serviceable parts in the system. Service can only be provided by a
trained TransMedics Service Representative. Any attempt to open any covers other than the covers intended
to be opened during system use can expose the user to potential physical or electrical hazards.
WARNINGS—
Opening the covers reserved for access by TransMedics service personnel could expose the user to electrical or physical hazards that
could cause serious injury or shock.
The OCS™ is intended for use only by qualified health care professionals who specialize in organ transplants and are trained in the
use of the OCS™.
Always follow your institutional procedures for use of aseptic procedures, for working inside a surgical field, and for handling and
disposing of blood-contaminated materials. Failure to do so can lead to biocontamination of the organ, the operating room
environment and personnel.
Do not use the system and accessories in the presence of explosive anesthetics.
Only a qualified TransMedics Service representative may service the system or any of its accessories. Any attempt by the user to
disassemble the OCS™ or any of its accessories may result in shock or serious injury and will void the warranty.
Cleaning and disinfection must be performed in a well-ventilated area to prevent inhalation of toxic fumes.
Failure to use personal protective equipment while cleaning and disinfecting may result in exposure to blood borne pathogens or
other potentially infective materials.
CAUTIONS—
Always use two people to lift or carry the system, which may weigh up to 45 kg (100 lb) without the organ, fluids, or the mobile base.
Users should avoid contact with the bellows in the Lung Perfusion Module while the OCS™ Lung Console is powered.
Wheel brakes are only meant to stop forward movement of the OCS™ Lung Console but the device can move backwards with the
brakes engaged.
Use only the black push handle to push the system, as using other surfaces could result in instability.
Do not use the push handle to lift the system. The handle is not designed to support the system weight. System damage or personal
injury may result if the push handle is used improperly.

Chapter 2: Safety Information
PN 100004070, REV 6 Page 15
2.4. Patient and Organ Safety
No System Contact with Patients: The system is intended only for preservation of an explanted organ. It is not
intended for direct contact with any patient.
WARNINGS—
The OCS™ Lung Console, the Lung Perfusion Module components and accessories, and the OCS™ Lung Solutions are not intended for
direct contact with any patient.
Always follow your institutional procedures for use of aseptic procedures, for working inside a surgical field and for handling and
disposing of blood-contaminated materials. Failure to do so can lead to biocontamination of the organ, the operating room
environment and personnel.
All parts of the Lung Perfusion Module and its sterile accessories are intended for single use only. To avoid the risk of
biocontamination, do not attempt to sterilize and reuse the Lung Perfusion Module or any of the sterile accessories.
CAUTIONS—
Avoid leaving the system in an uncontrolled temperature environment for longer than a few minutes. During such periods closely
monitor perfusate temperature and take remedial action if the temperature registers more than one degree over or under the
desired range. If necessary, adjust fluid temperature accordingly to accommodate external temperatures.
Use only accessories and supplies purchased from or recommended by TransMedics. Use of accessories and supplies other than
those supplied by or recommended by TransMedics may cause organ damage and will invalidate the TransMedics warranty. (This
manual details approved accessories and supplies as relevant to OCS™ operation.)
2.5. Component Safety
Inspection of sterile components before Use. Before use, aseptically open and inspect each component,
checking for any cracks, leaks, or other damage that might impact use.
Non Sterilizable. Do not attempt to sterilize the TransMedics system or any of its non-sterile components. The
Lung Perfusion Module and its sterile accessories are intended for single use only. Do not attempt to re-
sterilize any of these single use components.
TransMedics-Approved Accessories and Supplies Only. Use only accessories and supplies purchased from or
recommended by TransMedics. Use of accessories and supplies other than those supplied by or recommended
by TransMedics may invalidate the TransMedics warranty and may cause a safety hazard. (This manual details
approved accessories and supplies as relevant to system operation.)
Check Expiration Dates on Packaging. Always check the expiration date on each package. If the date has
expired, do not use the item.
2.6. Symbols Used on the System
Appendix A (Symbol Glossary) at the end of this document describes the symbols on the OCS™Lung System
packaging. Table 2-1 below shows and describes the symbols used on the Lung Perfusion Module and Console.

Chapter 2: Safety Information
PN 100004070, REV 6 Page 16
Table 2-1: Symbols Used on the Lung Perfusion Module and Lung Console
Symbol Meaning Symbol Meaning
Indicates On (only for a part of
the equipment)
Indicates Off (only for a part of the
equipment)
Run/Standby
Non-ionizing, electromagnetic radiation
Direct current Alternating current
Bronchoscope Port Level 1 ingress protection
Trachea Connector
Blood and Prime Solution Port
PA Connector
LA Return Line
Venous Sampling Port
Pulmonary Artery Pressure Port
Reservoir Injection Port Oxygenator Recirculation Line Connections
Venous Injection Port
Venous Oxygen Saturation/ Hematocrit
Probe
Arterial Sampling Port
Arterial Oxygen Saturation/ Hematocrit
Probe
Gas Intake Port
Venous Oxygen Saturation/ Hematocrit
Probe (on OCS™ Lung Console)
Metered Dose Inhaler Port
Arterial Oxygen Saturation/ Hematocrit
Probe (on OCS™ Lung Console)
Venous Oxygen Saturation/
Hematocrit Cuvette
Pump Flow Probe (on OCS™ Lung Console)
Arterial Oxygen Saturation/
Hematocrit Cuvette
Pump Flow Probe
Follow instructions for use
Pump Flow probe location (on LPM)
Drainage Bag Connection
Gas exchanger vent stopcock
NOTE—For detailed information on system status icons that appear on the Wireless Monitor and its display, see Chapter 5:
Wireless Monitor Overview.

Chapter 2: Safety Information
PN 100004070, REV 6 Page 17
2.7. Using Aseptic Technique
When using the OCS™ Lung System, you must use aseptic technique when performing any procedures that
involve the following:
•Touching the organ.
•Opening the organ chamber.
•Opening the sterile drape.
•Accessing the docked Wireless Monitor’s controls through the clear film of the TransMedics sterile
drape.
•Manipulating the lung inner wrap.
•Preparing and connecting solutions for use in the circuit.
•Collecting and filtering blood and transferring it to the reservoir.
•Making injections into the circuit.
•Sampling fluids from the circuit.
NOTE—Steps in which the sterile drape is opened, or in which the organ is touched, require use of surgical attire and strict
adherence to normal operating room procedure inside a sterile field.
Tasks that do not require aseptic technique include:
•Installing and removing batteries, gas cylinders, the Wireless Monitor, and probes.
•Other routine tasks that involve only the non-sterile components of the OCS™ or non-sterile
components outside the surgical field.
NOTE—After use, the entire Lung Perfusion Module and its one-time use accessories must be disposed of in accordance with
institutional procedures for disposal of blood-contaminated materials.
2.8. Moving the System
Read the following warnings and cautions before moving the system.
WARNINGS—
Failure to follow any of these precautions may result in injury and/or damage to the TransMedics OCS™ and its contents.
Disconnect the system power cord from the wall outlet and wind it snugly around the power cord wrap before moving the system.
When moving the system without installing the Mobile Base, use two people, one holding the right lift handle and one holding the
left lift handle.
Before transporting the OCS™ in a vehicle, strap it securely in place.
During transport, position the OCS™ Lung Console so that it never sits at an angle of greater than 15 degrees from vertical.

Chapter 2: Safety Information
PN 100004070, REV 6 Page 18
CAUTIONS—
Angles greater than 15 degrees may disrupt fluid paths in the Lung Perfusion Module and lead to system malfunction.
Never use the push handle to lift the system during a move, with or without the Mobile Base attached.
Only use the black push handle to roll the OCS™ Lung Console as using other surfaces could result in instability.
Wheel brakes are only meant to stop forward movement of the OCS™ Lung Console but the device can move backwards with brakes
engaged.
TransMedics recommends attention to outside temperature; low temperatures can accelerate battery depletion. When moving the
system outside, be sure to keep the OCS™ top cover in place to conserve energy.
During transport, do not subject the OCS™ to vibration levels higher than those to which a patient can be safely exposed. Excessive
vibration may disrupt fluid paths in the Lung Perfusion Module and lead to system malfunction.
During transport, avoid sudden stops, turns, and reversals in direction that might subject the OCS™ to high lateral acceleration.
When moving the system with the Mobile Base attached:
•Make sure the system is properly mounted and latched on the Mobile Base.
•Make sure the system wheel locks are disengaged and that the wheels are free to rotate prior to
moving the system.
•If you must move the system up or down ramps with an incline of more than 5 degrees, use two
people to move the system.
•Do not use the push handle to lift the system.
2.9. Storing the System Between Uses
Follow these guidelines to store your system between uses:
•Before storing, clean the system and probes as described in Chapter 7: Cleaning and Maintaining
the OCS™.
•Store the system in a clean, dry area away from traffic.
•Press to set the system to Standby Mode.
•Connect the OCS™ power cord to an active AC power source and ensure the On/Off switch remains
in the On position to recharge the batteries.
•Wrap the excess power cord to eliminate interference with traffic in the area.
•Store only in areas that meet the temperature and humidity conditions specified in Table 8-2,
“OCS™: Electrical and Physical Specifications.”
•Store the flow and SO2/Hematocrit sensors within the Console, connected to the system.
•Set the wheel locks.

Chapter 2: Safety Information
PN 100004070, REV 6 Page 19
2.10. Shipping, Handling and Storage Requirements
Figure 2-1: Shipping, Handling, and Storage Requirements Symbols
Unless otherwise noted the OCS™ and its accessories have the following shipping, handling and storage
requirements:
1. 10% to 95% Humidity Limitation
2. 50 to 106 kPa Atmospheric Pressure Limitation
3. -20 to 50°C Ambient Temperature
4. If so marked the package must only be oriented the indicated side up
5. Keep away from sunlight
6. Fragile, handle with care
7. Handle with care
8. Keep away from rain
2.11. Periodic Safety Tests
Table 2-2 is provided for institutions that require periodic safety checks to assure that systems continue to be
safe. The table lists the maximum allowed values.
Table 2-2: Safety Test Guidelines
Test Maximum Allowed (IEC 60601-1)
Earth Leakage Current 300 μA Normal Condition (IEC 60601-1 Fig 16)
1000 μA Single Fault Condition (IEC 60601-1 Fig 16)
Enclosure Leakage Current 100 μA Normal Condition (IEC 60601-1 Fig 18)
300 μA Single Fault Condition (IEC 60601-1 Fig 18)
Earth Continuity 0.1 ohms (excluding supply cord)
CAUTION—The OCS™ relies on a proper earth ground to provide safe operation when operating on AC power. The ground
integrity should be checked, at a minimum, annually to assure that proper grounding exists.

Chapter 2: Safety Information
PN 100004070, REV 6 Page 20
2.12. Explosion Hazard
WARNING—Do not use the system and accessories in the presence of explosive anesthetics.
2.13. Electromagnetic Compatibility
The OCS™ meets the following electromagnetic compatibility standards:
•IEC 60601-1-2 (International and U.S.)
•EN 60601-1-2 (Europe)
For more information on electromagnetic compatibility, see Chapter 8: System Specifications.
2.14. Electromagnetic Interference (EMI)
Though the OCS™ has been designed to resist electromagnetic interference, the proliferation of radio-
frequency transmitting equipment and other sources of electrical noise in the health care environment may
result in disruption of performance. EMI interference is indicated by erratic readings, cessation of operation or
other operational problems. If these symptoms occur, the site of operation should be surveyed to determine
the source of disruption and action taken to eliminate the source. If assistance is required, contact
TransMedics.
To help identify the source of electromagnetic interference, ask the following questions:
•Is the interference intermittent or constant?
•Does the interference occur with one sensor only, or with several sensors?
•Is the interference present if the system is moved to a different location in the facility? For
example, moving the cable or other medical equipment away from the system can reduce
electromagnetic interference.
Please answer these questions before contacting your service representative. The answers will help a service
representative determine if the problem is in the system or in the environment.
2.15. Handling Batteries
Your system contains three user-replaceable system batteries in the OCS™ Lung Console and one non-user
replaceable battery in the Wireless Monitor.
NOTE—The Wireless Monitor battery can be serviced and replaced only by qualified TransMedics Service Personnel.
To use the OCS™ Lung Console to recharge the system and Wireless Monitor batteries, the system must be
connected to AC power and the On/Off switch must be in the On position. The OCS™ Lung Console cannot
recharge the Wireless Monitor battery unless the Wireless Monitor is docked.
CAUTION—Each battery includes rechargeable lithium ion cells. Lithium is a highly reactive element which reacts violently when
mixed with water, leading to possible battery leakage, smoke, and fire. Batteries must be handled, stored and disposed of with care
to prevent physical damage to the battery and to meet the environmental requirements specified.
Table of contents
Other TransMedics Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual