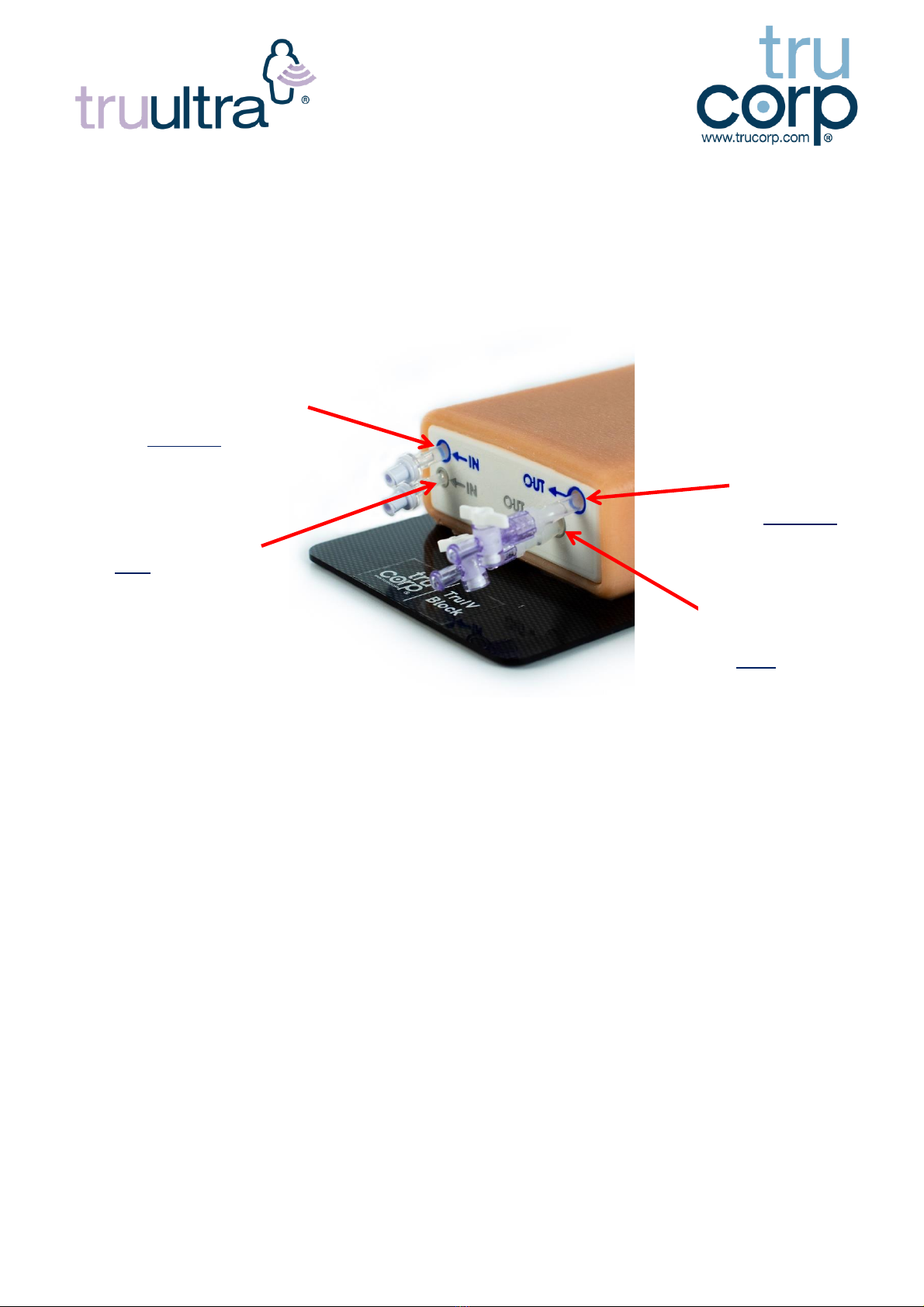
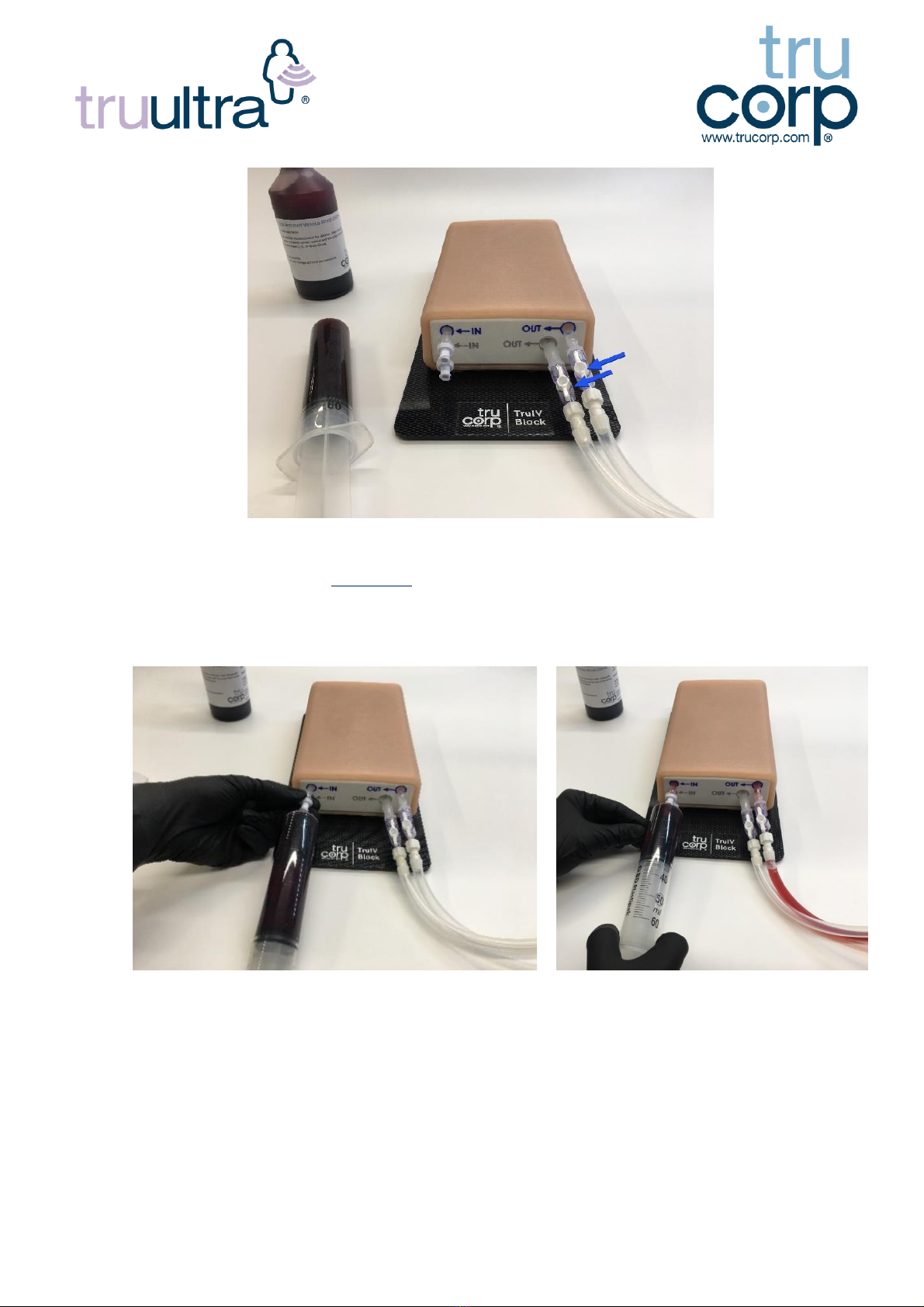
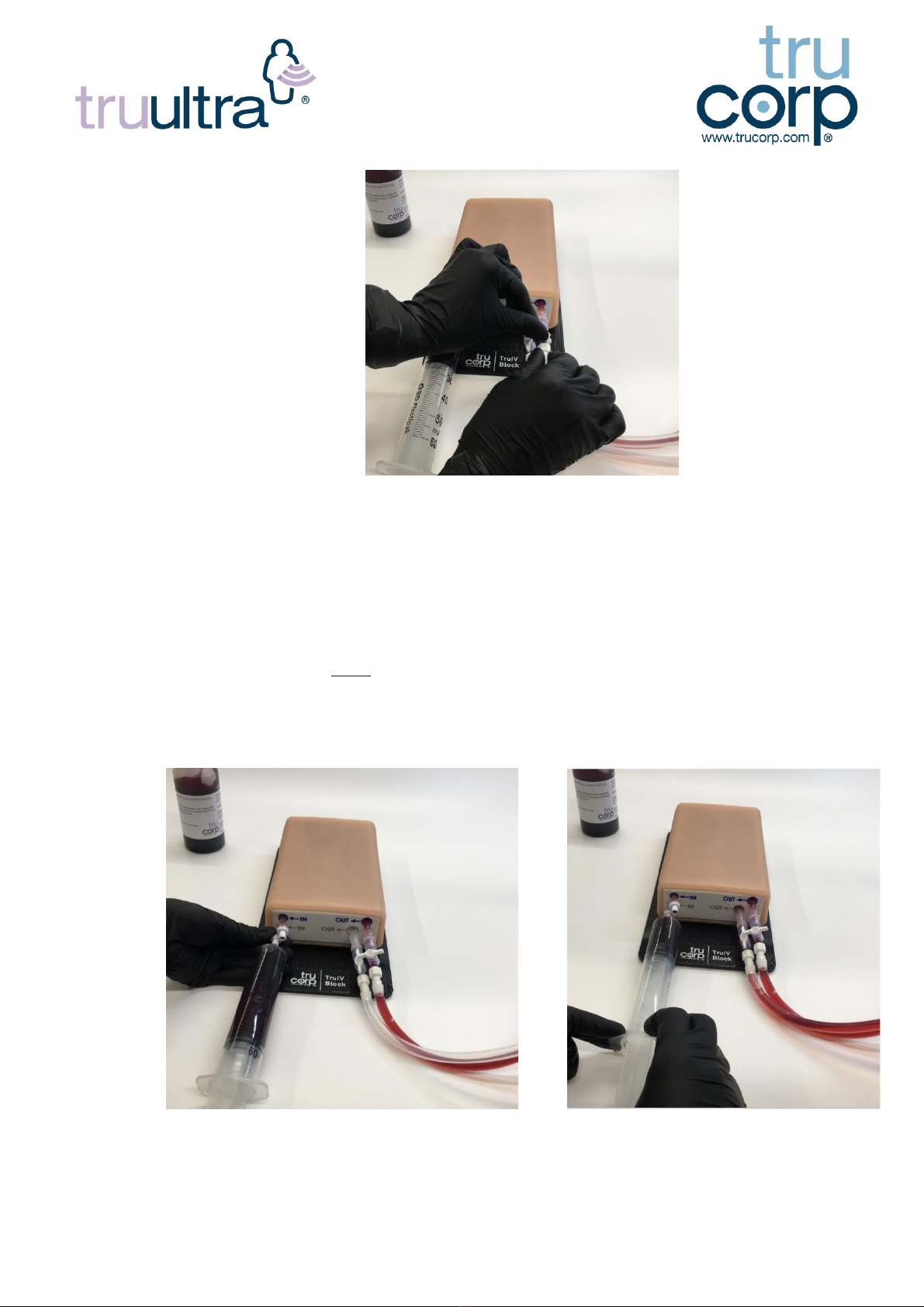
10
Store the product in clean, dry conditions away from heat and direct sunlight; avoid contact
with metals, solvents, oils or greases and strong detergents. When the product is not in use
please store in the black carrier case provided.
Gently wash the ultrasound insert after use. Please use alcohol wipes, warm soapy water or
similar, until all visible foreign matter and residue are removed.
Mild detergents or enzymatic cleaning agents may be used on the insert in accordance with
the manufacturer's instructions and at the proper dilution. The detergent must not contain
skin or mucous membrane irritants.
Please do not use any of the following when cleaning the AirSim product range
•Germicides, disinfectants, or chemical agents such as glutaraldehyde (e.g. Cidex®),
•Ethylene oxide, phenol-based cleaners or iodine-containing cleaners
In response to the recent COVID-19 pandemic, we recommend this additional step to ensure
the product is fully sanitised:
Use alcohol spray (minimum 75%) and wipe off. Repeat this for 3-4 times to ensure to kill
the virus completely.
Warranty
TruCorp warrants this unit to be free of defects in materials and workmanship and to give
satisfactory service for a period of 1-year from the date of delivery. This ensures that our
customers receive maximum coverage on each product. If the unit should malfunction it
must be returned to the factory for evaluation. Upon examination by TruCorp, if the unit is
found to be defective it will be repaired or replaced at no charge.
TruCorp will pay for the freight/delivery and the actual parts needed free of charge if any
part of the product fails within the 1-year period.
However, these warranties are VOID, if; the unit shows evidence of having been tampered
with or shows evidence of having been damaged by excessive heat, the use of sharp
instruments, misapplication, misuse or other operating conditions outside of TruCorp’ s
control. Components which wear or are damaged by misuse are not warranted and will be
charged for if repair has been approved. Please ensure to strictly follow the recommended