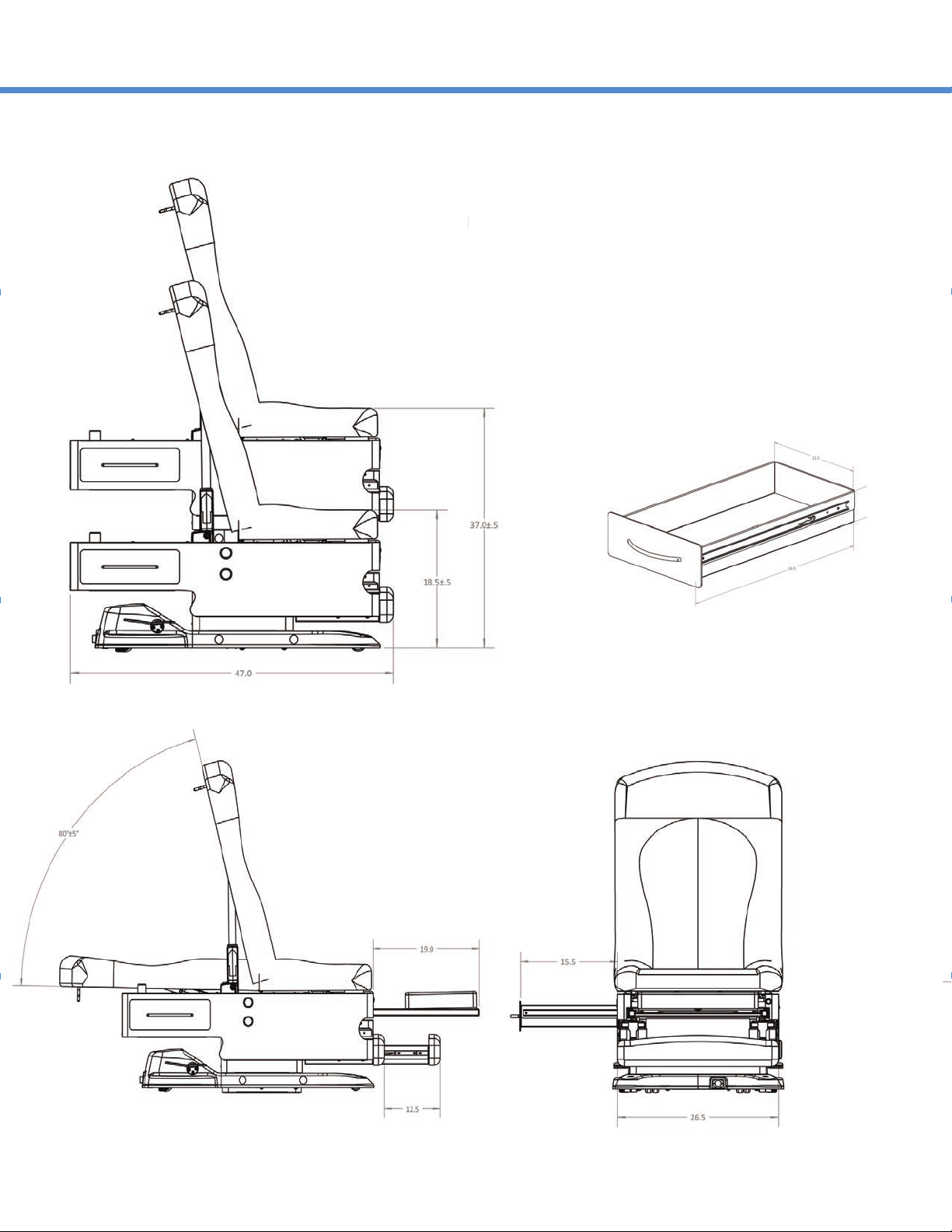
FEATURES AND OPERATION
REMOVABLE UPHOLSTERED TOP (INSTALLATION)
REMOVABLE UPHOLSTERED TOP (REMOVAL)
STEP 1:
Carefully lay upholstered top on
table, aligning black slide latches
on underside of upholstery with
cutouts in support pan.
slide latches may not be perfectly
aligned when upholstery is resting
on the support pan. Proceed to
next step.
If latches are not properly
aligned, slightly raise seat section
and maneuver upholstery slightly
until all latches align properly.
Raise the back section of the
upholstery and support pan
(4070-650:) by squeezing the
manual release lever and gently
lifting upward. (4040-650:) by
using hand or foot control.
Using your index finger, slide all
four black slide latches toward the
center of the support pan until
fully engaged. Lower the back
section.
Raise the foot section of the
upholstery and support pan, and
repeat the step five for the
remaining four black slide latches.
Lower the foot section.
Grasp slide latch with thumb and
forefinger.
Pull the slide latch toward you
(away from the metal pan).
While pulling toward you (away
from the pan), slide the latch to
the unlocked position.
Allow the latch to return to its
normal position. Repeat for all
latches.
STEP 3:
STEP 5:
STEP 2:
STEP 4:
STEP 1:
STEP 3:
STEP 2:
STEP 4:
STEP 6:
Page 9
3-00077-0318A