The indicated use is for treatment of ocular damage and disease using photobiomodulation,
including inhibition of inflammatory mediators, oedema or drusen deposition, improvement of wound
healing following ocular trauma or surgery, and increase in visual acuity and contrast sensitivity in
patients with degenerative diseases such as dry age-related macular degeneration.
Indications for Use
Introduction .................................................................................................................................................................................................
Valeda Light Delivery System Description ...............................................................................................................................
Valeda Light Delivery System Operation .................................................................................................................................
Labelling Symbols ....................................................................................................................................................................................
Indications for Use .................................................................................................................................................................................
Contraindications for Use .................................................................................................................................................................
Important Safety Instructions .........................................................................................................................................................
Installation ...................................................................................................................................................................................................
Operating Instructions .........................................................................................................................................................................
Valeda Treatment Credits ...............................................................................................................................................................
Valeda Settings ......................................................................................................................................................................................
Cleaning .....................................................................................................................................................................................................
Disposal ......................................................................................................................................................................................................
Maintenance ...........................................................................................................................................................................................
Troubleshooting .....................................................................................................................................................................................
Technical Specifications .................................................................................................................................................................
Glossary ......................................................................................................................................................................................................
Guidance and Manufacturer’s Declarations .....................................................................................................................
1. Introduction
This manual provides the indications, contraindications, instructions for use, warnings, cautions
and precautions for the Valeda® Light Delivery System. Carefully read this manual in its entirety
before using the Valeda Light Delivery System. Failure to follow these instructions may result in
improper use of the system.
Terminology – within this User Manual, the words “Valeda” and “System” are used to identify
collectively all components (and accessories) of the Valeda Light Delivery System. For questions
regarding Valeda, please contact LumiThera®.
2. Valeda Light Delivery System Description
Overview
Valeda is a multi-wavelength light emitting diode (LED) system designed for eye care professionals
to use in the treatment of the eye with photobiomodulation (PBM). Valeda will provide a pre-set
treatment of PBM to the patient’s eye and retinal tissue through the open and closed eyelid.
Valeda uses three LEDs to generate 590, 660 and 850 nm light, which is conditioned through a series
of optics to produce a uniform, noncoherent beam of 30 mm diameter at the plane of treatment.
The beam is directed by adjustable mirrors to allow treatment of either eye without repositioning
the patient.
The operator inteace (Figure 1) consists of a touchscreen, start and stop push buttons, and a
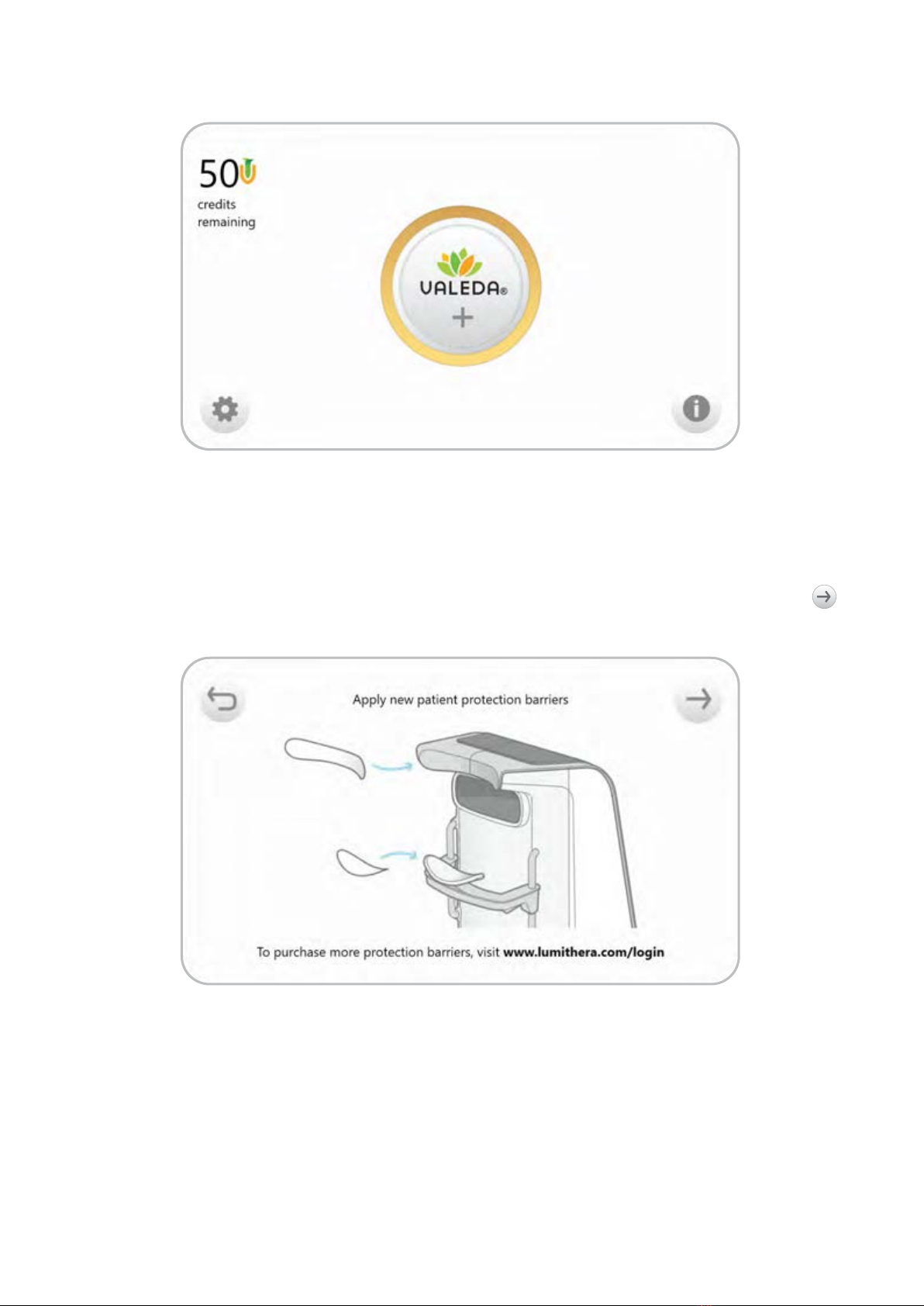
joystick to centre the beam on the patient’s eye. A USB port is used to load Valeda Treatment
Credits into the system.
Contents
1
1
3
4
5
5
6
7
7
17
19
20
20
20
21
23
24
25
1
English (UK)
1.
2.
3.
4.
5.
6.
7.
8.
9.
10.
1 1.
12.
13.
14.
15.
16.
17.
18.