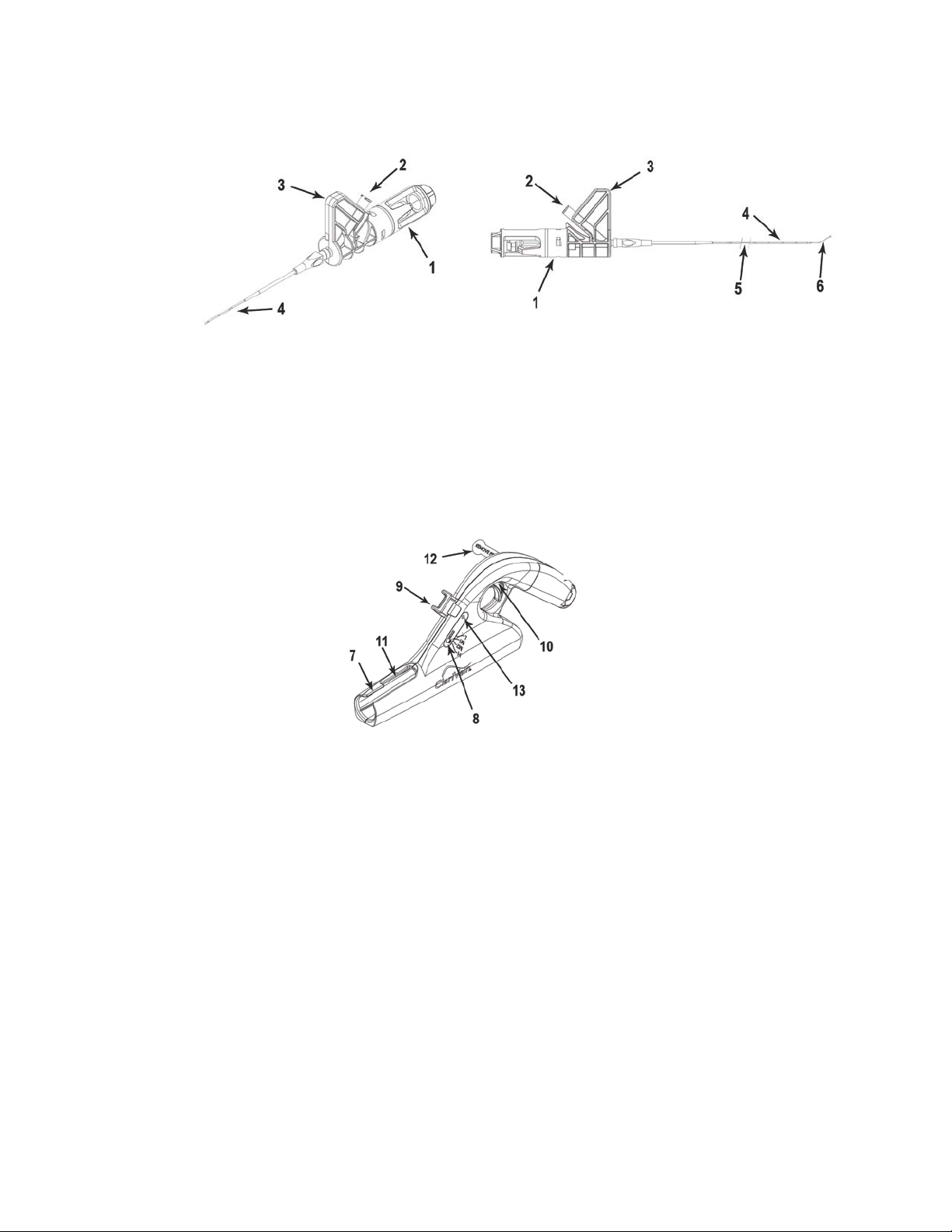
CONTRAINDICATIONS
The ClariVein®IC is not intended for use in the following:
•Coronary and cerebral vasculature
•Pulmonary vasculature
•Diseased and atherosclerotic arteries
•Infusion of blood and blood products
WARNINGS
•This product should be used by physicians that have a thorough understanding of
intravascular ultrasound, angiography, peripheral vascular procedures and anatomy.
•Prior to use, carefully examine the ClariVein®IC and package contents included with
ClariVein® IC and verify they have not been damaged during shipment. If the components
show any sign of damage DO NOT USE.
•After use, dispose of the product per institutional protocol.
•Due to the risk of exposure to HIV or other blood borne pathogens, health care workers should
always use standard blood and body fluid precautions in the care of all patients. Sterile
techniques should be strictly adhered to during any handling of the device.
•Do not modify the device. To do so could result in injury, illness, or death.
CAUTIONS
•Do not use the ClariVein®IC in patients contraindicated for endovascular procedures.
•Do not use without completely reading and understanding the instructions for use.
NOTE: Packaging contents contains no medications. Prior to use, carefully read and
understand the respective manufacturer’s instructions for procedural accessory devices and
solutions intended for use including warnings, cautions, potential side effects and
contraindications.
•Before using ClariVein®IC, verify proper function and integrity of the device.
•Refer to package label for expiry date and do not use after expiration.
•Rotation of the ClariVein®IC Dispersion Wire is internally powered via a 9V DC battery. Prior
to use of the device, remove the Battery Terminal Insulator Tab by pulling tab away from
device.
•The integral 9V, DC battery is not intended to be either removed or replaced.
•Do not use in the presence of a flammable anesthetic mixture with air or with oxygen or nitrous
oxide in order to reduce any potential of static discharge or other ignition hazards.
•Select an appropriately sized vascular access device.
•Failure to use a compatible access device may result in damage to the device or cause patient
injury.
•Confirm syringe and Check Valve connections. Do not use if a leak persists.
•Manipulate the catheter in the vessel only under vascular imaging.
•Do not exert excessive force when withdrawing or advancing catheter. If resistance is
encountered, determine if remedial action is necessary. Failure to do so may result in device
damage or patient injury.
•Utilize vascular imaging such as ultrasound to confirm that the catheter tip is in the desired
location before activation of Dispersion Wire rotation.
____________________________________________________________________
ClariVein®is a registered trademark of Vascular Insights™, LLC in the United States.
Page 2