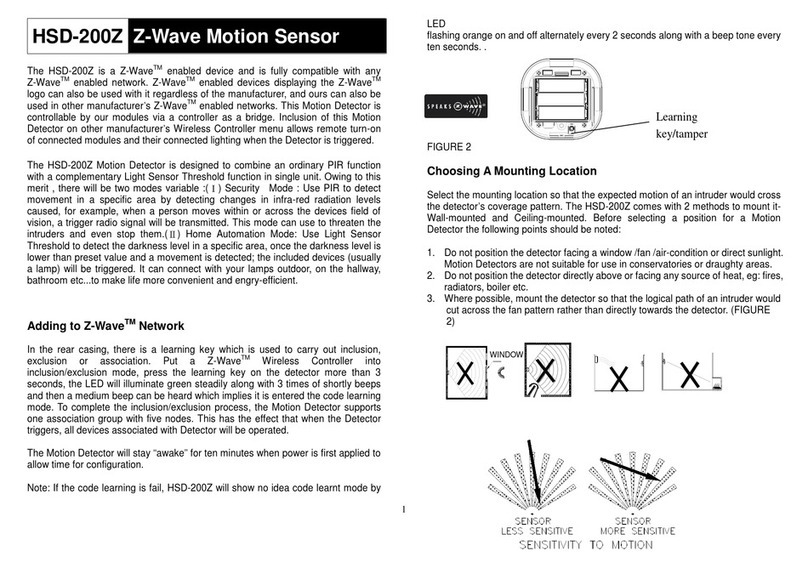
2
1 General information
VEGAFLEX 83 •
43113-EN-140114
1 General information
FDA stands for Food and Drug Administration, a U.S. authority.
Among other things, this authority issues a regulation on the use of
product-contacting materials in the pharmaceutical, food and bever-
age and cosmetics industries (Code of Federal Regulations CFR).
Always use a suitable, FDA-approved process seal.
The USP (United States Pharmacopeia) is an ocial authority issuing
the quality standards for drugs and medical products. USP checks
and documents also food additives as well as materials and their ef-
fect on living tissue.
Regulation (EC) No. 1935/2004 of 27/10/2004 aims at securing a
protective level for human health and the consumer of objects and
materials coming into contact with foodstus. The main focus of the
regulation is ensuring that good manufacturing practices are main-
tained. For us, the rst aspect of good manufacturing means realizing
the goal of ensuring that components potentially coming into contact
with foodstus are designed so that under foreseeable conditions a
migration of constituent substances is largely avoided or does not oc-
cur in quantities that endanger human health or cause unacceptable
changes in the composition or organoleptic properties of the material.
We fulll these general requirements by purchasing components
delivered with proof of compliance with FDA requirements and, when
it comes to materials not subject to explicit FDA requirements, by
obtaining a "No Objection Letter" from the Public Health Service of
the Food and Drug Administration as well as requesting the expert
opinion of independent (accredited) laboratories and organizations.
Under the second aspect of good manufacturing practice we
understand ensuring the traceability of components and products
potentially coming into contact with foodstus throughout all stages of
manufacturing and sales. This is guaranteed by our quality manage-
ment system according to ISO 9001 und ISO 14001.