ENGLISH • 5
IMPORTANT
SAFEGUARDS
CARE & SAFETY INFORMATION
• Do not use while bathing or with wet hands.
• Do not use unit where aerosol products are being used or where oxygen, nitrous oxide or
anesthetics are being administered.
• The medicine liquid of usage and capacity must apply to doctor’s advice.
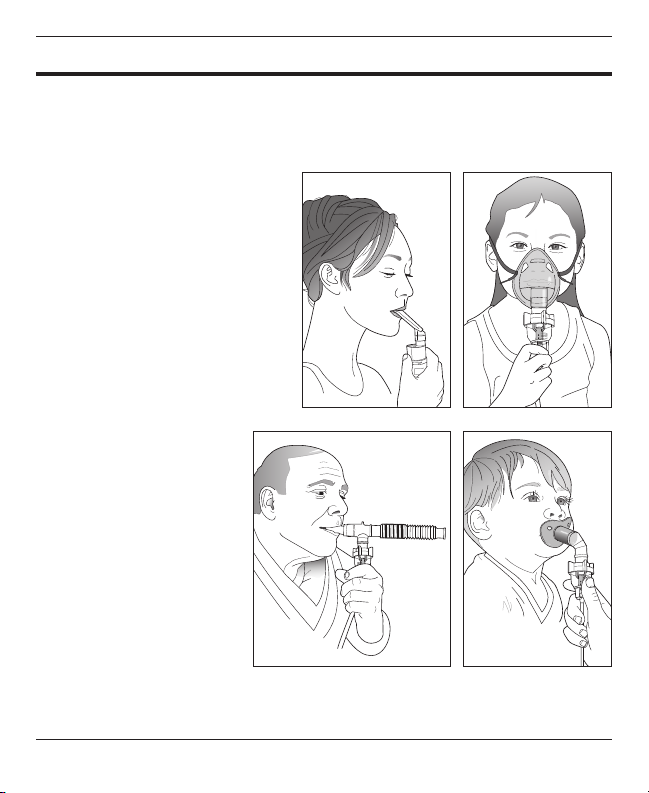
• Do not allow the nebulized medicine to come in contact with your eyes.
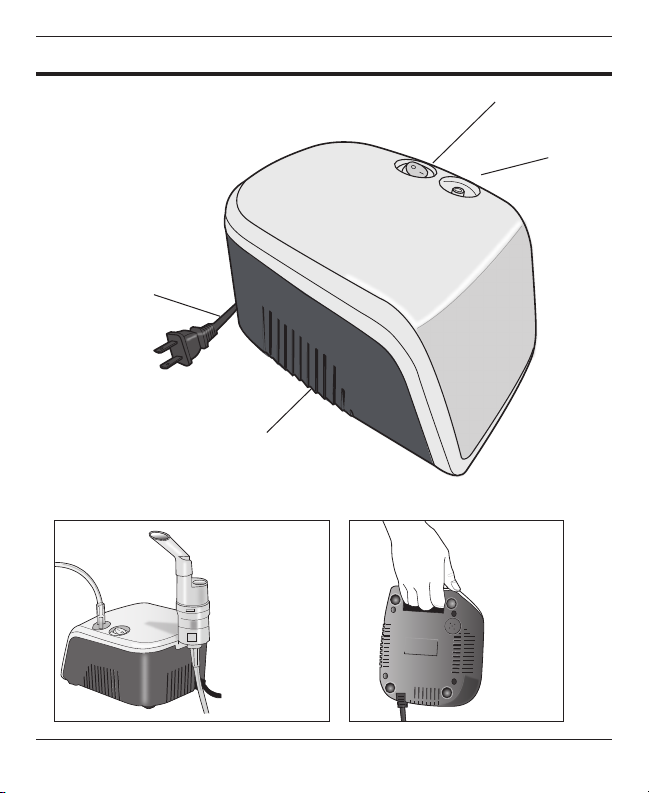
• Always ensure that the air vents are not blocked or obstructed in any way.
• If the nebulizer is not functioning correctly, if it makes unusual sounds, or if during use
you feel pain or discomfort, immediately stop use.
• Do not unplug the air tube during the treatment, otherwise may get injured because of
the eect of pressure.
• Do not bend the air tubing of the unit where the air tube may be obstructed.
• Always turn o and unplug the unit after use.
• Do not expose the unit to direct sunlight, heated or hot surfaces, humid environments,
extreme temperatures, strong static electricity or electromagnetic waves.
STORAGE CAUTIONS AND WARNINGS
• Do not store the unit in direct sunlight, high temperatures or humidity.
• Keep the unit out of reach of children.
• Always keep the unit unplugged when not in use.
CLEANING CAUTIONS AND WARNINGS
• Clean all necessary parts after each treatment as instructed in this manual; if the unit has
not been used for an extended period, all parts should be cleaned prior to use.
• Disconnect the unit from the electrical outlet before cleaning.
• Never immerse the unit in water to clean as it may damage the unit.
• Do not use abrasive cleaners.