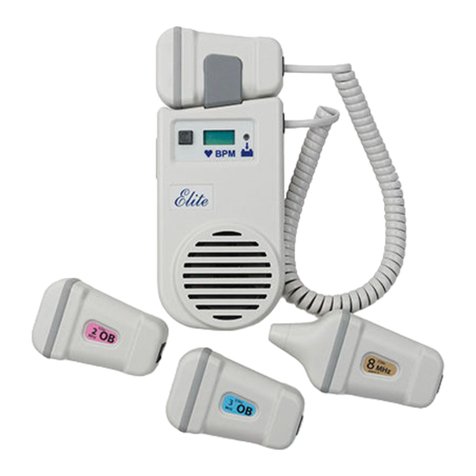
Nicolet system warranty
VIASYS NeuroCare warrants that each product we sell you shall conform to its product speci-
fications as defined in the user documentation.
If the product does not function as warranted during the warranty period, we will repair or
replace it without charge. If in our judgment we are unable to do so, you may return it to us and
we will refund your money.
Warranty period
The warranty period is stated in the product user documentation. If you install the product, the
warranty period begins on the date of invoice. If we install the product, the warranty period
begins on the date of installation but will begin no later than 30 days from the date of invoice.
The warranty period for products sold outside the U.S.A. and Canada is 12 months from the
date of installation or 14 months from the date of shipment, whichever is less.
Limit of warranty
Misuse, accident, modification, unsuitable physical or operating environment, improper main-
tenance, or damage caused by a product for which we are not responsible may void the war-
ranty.
Certain components may have separate warranty periods as stated in the product user docu-
mentation. Consumables are not covered under warranty.
THIS WARRANTY REPLACES ALL OTHER WARRANTIES, EXPRESSED OR
IMPLIED, INCLUDING THE IMPLIED WARRANTIES OF MERCHANTABILITY AND
FITNESS FOR A PARTICULAR PURPOSE AND ANY OTHER OBLIGATIONS OR LIA-
BILITIES ON THE PART OF VIASYS NeuroCare WHETHER IN CONTRACT, WAR-
RANTY, NEGLIGENCE OR OTHERWISE. VIASYS NeuroCare SHALL NOT BE LIABLE
FOR AND DISCLAIMS ALL CONSEQUENTIAL, INCIDENTAL AND CONTINGENT
DAMAGES.
Items not covered by warranty
We do not warrant uninterrupted or error-free operation of a product.
We provide certain non-VIASYS NeuroCare products on an ‘as is’ basis.
Non-VIASYS NeuroCare manufacturers or suppliers may provide their own warranties to you.
Separate software warranty is provided with software user documentation.