2
experience and/or a lack of knowledge, unless they are supervised
by a person who has responsibility for their safety or they receive
instructions from this person on how to use the device. Children
should be supervised around the device to ensure they do not play
with it.
Do not allow the electrodes of the device to come into contact with
other conductive parts (including earth).
Do not use the device with persons with sensitive skin or allergies.
Do not store the device in the following locations: locations in which
the device is exposed to direct sunlight, high temperatures or levels
of moisture, or heavy contamination; locations near to sources of
water or fire; or locations that are subject to strong electromagnetic
influences.
Do not swing the device with the strip, which may result in injury.
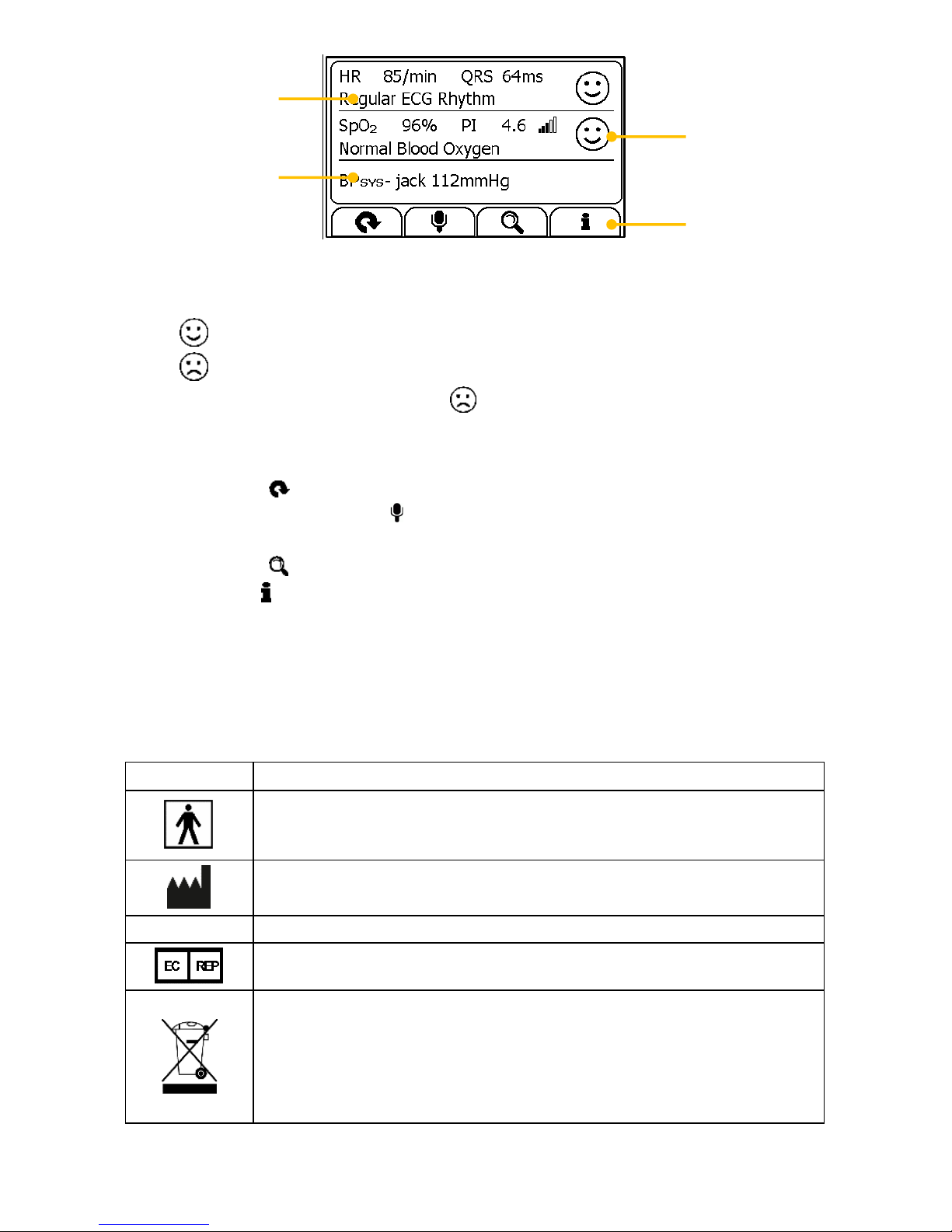
This device displays changes in the heart rhythm and blood
oxygenation etc. which may have various different causes. These
may be harmless, but may also be triggered by illnesses or diseases
of differing degree of severity. Please consult a medical specialist if
you believe you may have an illness or disease.
Vital signs measurements, such as those taken with this device,
cannot identify all diseases. Regardless of the measurement taken
using this device, you should consult your doctor immediately if you
experience symptoms that could indicate acute disease.
Do not self-diagnose or self-medicate on the basis of this device
without consulting your doctor. In particular, do not start taking any
new medication or change the type and/or dosage of any existing
medication without prior approval.
This device is not a substitute for a medical examination or your
heart or other organ function, or for medical electrocardiogram
recordings, which require more complex measurements.
It is not possible to use this device to diagnose illness or diseases.
This is exclusively the responsibility of your doctor.
We recommend that you record the ECG curves and other
measurements and provide them to your doctor if required.
Changes or modifications to this unit not expressly approved by the
party responsible for compliance could void the user’s authority to
operate the equipment.