3
Wagner & Guder Medical GmbH
issue May 2021
Direction for use, cubeLITE
Table of contents
SAFETY INSTRUCTIONS................................................................................................ 4
Description of symbols...................................................................................................... 4
Application area................................................................................................................ 5
Informations for the operator............................................................................................. 6
REQUIREMENTS FOR OPERATION............................................................................... 8
Before the first operation................................................................................................... 8
Before the operation ......................................................................................................... 8
During the operation ......................................................................................................... 8
Liability and warranty........................................................................................................ 8
POSITIONING AND INSTALLATION INSTRUCTIONS.................................................... 9
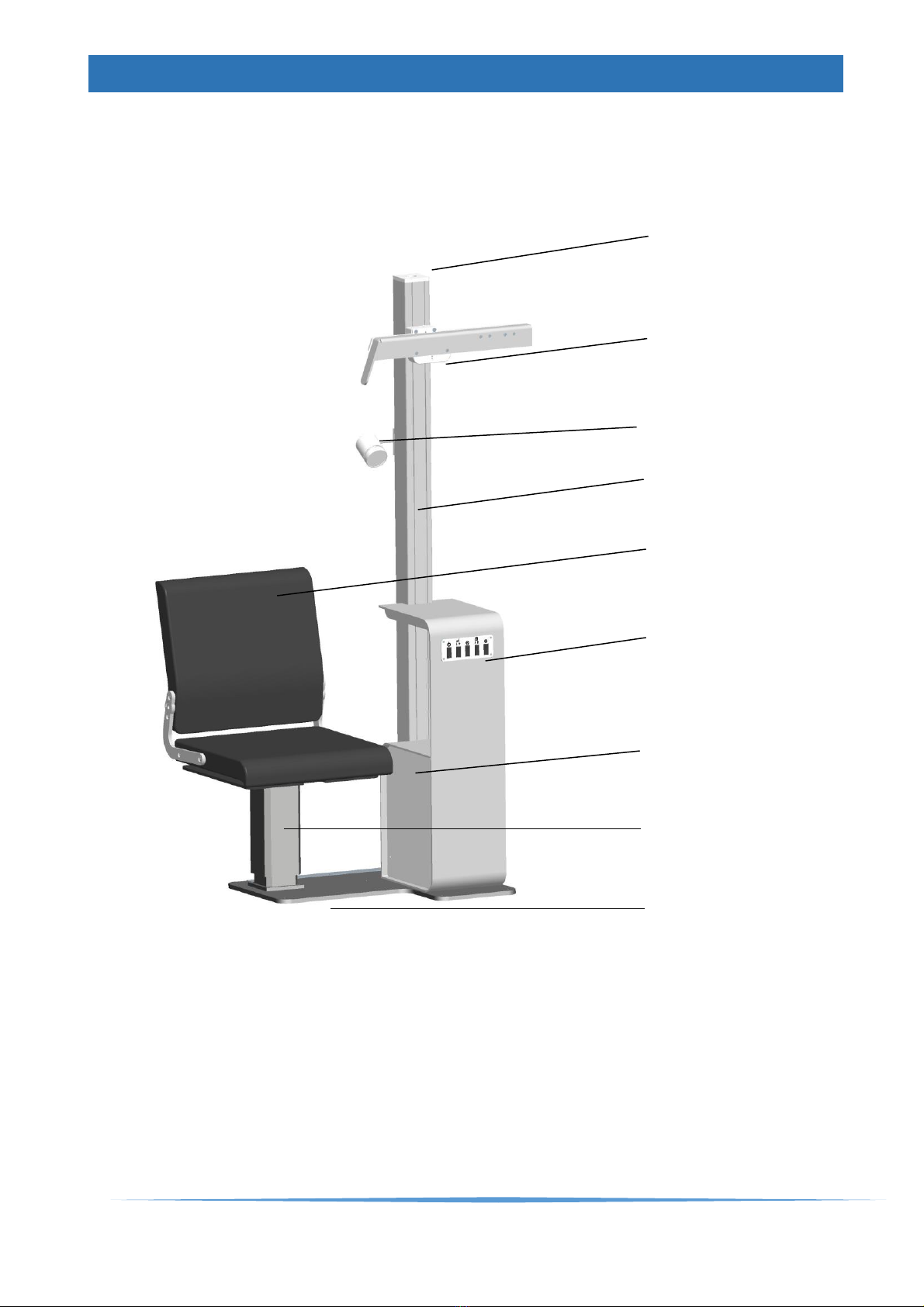
GENERAL CONSTRUCTION CUBELITE........................................................................10
Phoropter arm Type II......................................................................................................11
Patient chair.....................................................................................................................12
OPERATION ...................................................................................................................13
Electrical connection........................................................................................................13
Control panel...................................................................................................................14
Cleaning ..........................................................................................................................15
Maintenance/ Technical service.......................................................................................15
MAINTENANCE OF THE DEVICE...................................................................................16
Safety check....................................................................................................................16
Change of the main fuses................................................................................................16
ENVIRONMENTAL CONDITIONS...................................................................................17
PATIENTS SURROUNDING ...........................................................................................18
TECHNICAL DATA..........................................................................................................19
WARRANTY AND WASTE DISPOSAL............................................................................20
MANUFACTURER'S DECLARATION..............................................................................21
Eliminating electromagnetic interference..........................................................................25
PURCHASE ORDER DETAILS.......................................................................................27
Spare parts......................................................................................................................27