8
11) How to Measure
Prior to use on a patient, place a barrier sleeve over the Osstell Beacon.
Thebarriersleevehelpspreventcross-contaminationandhelpskeep
dental composite material from adhering to the surface of the instru-
ment tip and body, and discoloration and degradations from cleaning
solutions.
Note:
• Barrier sleeves are single patient use only.
•Discardusedbarriersleevesinstandardwasteaereachpatient.
• Do not leave barrier sleeves on the instrument for extended periods.
• See below for recommended barrier sleeves.
TIDIshield, Art no: 21021, Art no: 20987. www.tidiproducts.com
PremiumPlus: 123, Small short 123, Small
Please also see additional recommended barrier sleeves on:
osstell.com/get-started-beacon-us.
• The Osstell Beacon instrument must be cleaned and disinfected with
appropriatecleaninganddisinfectantuidsaereachpatient.See
section 15) Cleaning and Maintenance for acceptable agents.
Arstmeasurementshouldbetakenatimplantplacementtogeta
baseline for future measurements throughout the healing process.
Beforethenalrestoration,anothermeasurementistakenwhichmakes
it possible to observe the stability development of the implant.
ItisrecommendedtomeasureinbothBuccal-LingualandMesial-
Distaldirectiontondtheloweststability.Therefore,theOsstellBeacon
prompts the user to measure in both these directions.
We recommend you studying the more detailed information (videos and
quick guides) available on osstell.com/get-started-beacon-us,
to utilize the full functionality of your Osstell Beacon.
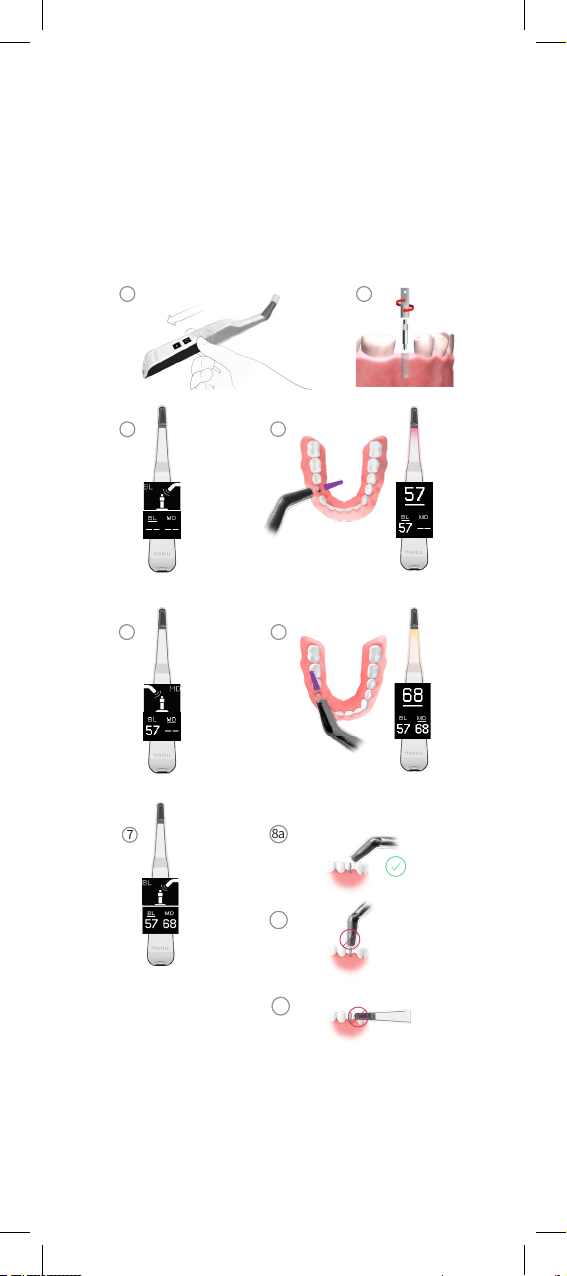
1. Activate the Osstell Beacon by picking it up. The instrument will
start-upandaershowingthebatterystatus,theinstrumentwillbe
ready for measurement in the BL (Buccal – Lingual) direction, which is
prompted in the upper display as well as optimal angle of the instru-
ment tip towards the Smartpeg.
2. Place a barrier sleeve over the Osstell Beacon instrument. See g 1.
3. Place the SmartPeg into the SmartPeg Mount. The SmartPeg is
magnetic, and the SmartPeg Mount will hold the SmartPeg.
See g 2. Attach the SmartPeg to the implant or abutment by screw-
ingtheSmartPegMountusingngerforceofapproximately4-6Ncm.
Donotover-tighten,toavoiddamagingtheSmartPegthreads.
4. Bring the instrument inside the mouth and hold the instrument tip
close(2-4mm)tothetopoftheSmartPegwithouttouchingit.
Hold the tip at approx. 45º angle towards the SmartPeg top as indicat-
ed in the upper display and shown in g 3 and g 8a. Do not measure
in the ways indicated in g 8b or g 8c.
An audible sound indicates when measurement has started, and
measured data will be shown in the upper display combined with a
colored light indication below the instrument tip. See g 4. Bring the
instrument out of the mouth to clearly read the ISQ value and the
colored indication.
The measured ISQ values will be displayed in the upper display for a
couple of seconds and then switch to indicate ready for measurement
inMesial-Distaldirection.See g 5.
Note! Do not bring the instrument back in the mouth until the display
has switch to the next direction.