2
Contents
1 Product Introduction .............................................................................................................. 4
1.1 Introducing S-Patch Ex Wearable ECG Patch .................................................................. 4
2 Indications for Use ................................................................................................................. 5
3 Safety Information ................................................................................................................. 6
3.1 Contraindications.......................................................................................................... 6
3.2 Warnings ..................................................................................................................... 6
3.2.1 General.............................................................................................................. 6
3.3 Cautions ...................................................................................................................... 6
3.3.1 General.............................................................................................................. 6
3.3.2 MR-unsafe ......................................................................................................... 6
3.3.3 EMC .................................................................................................................. 7
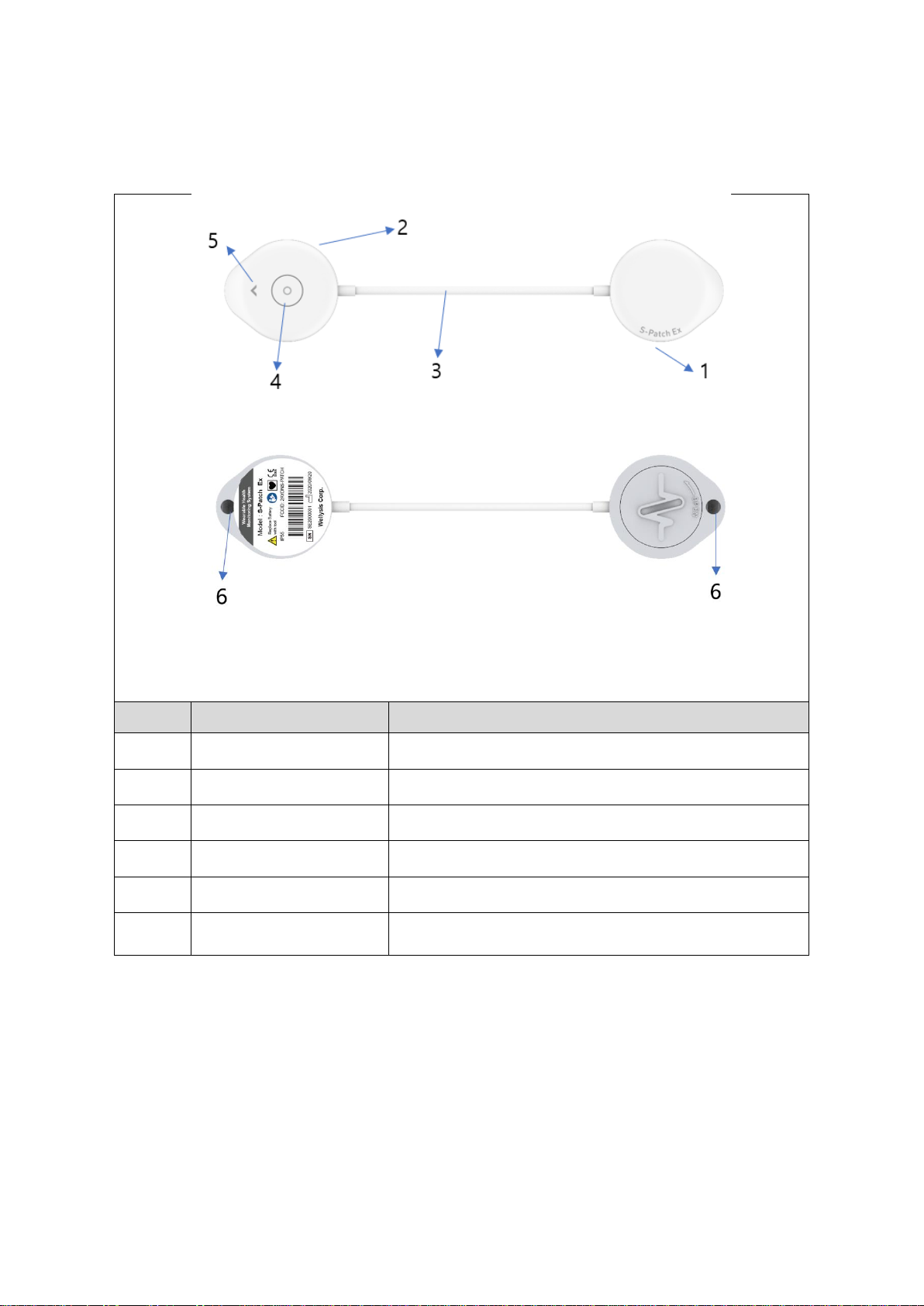
4 S-Patch Ex Components ......................................................................................................... 8
4.1 Components list ........................................................................................................... 8
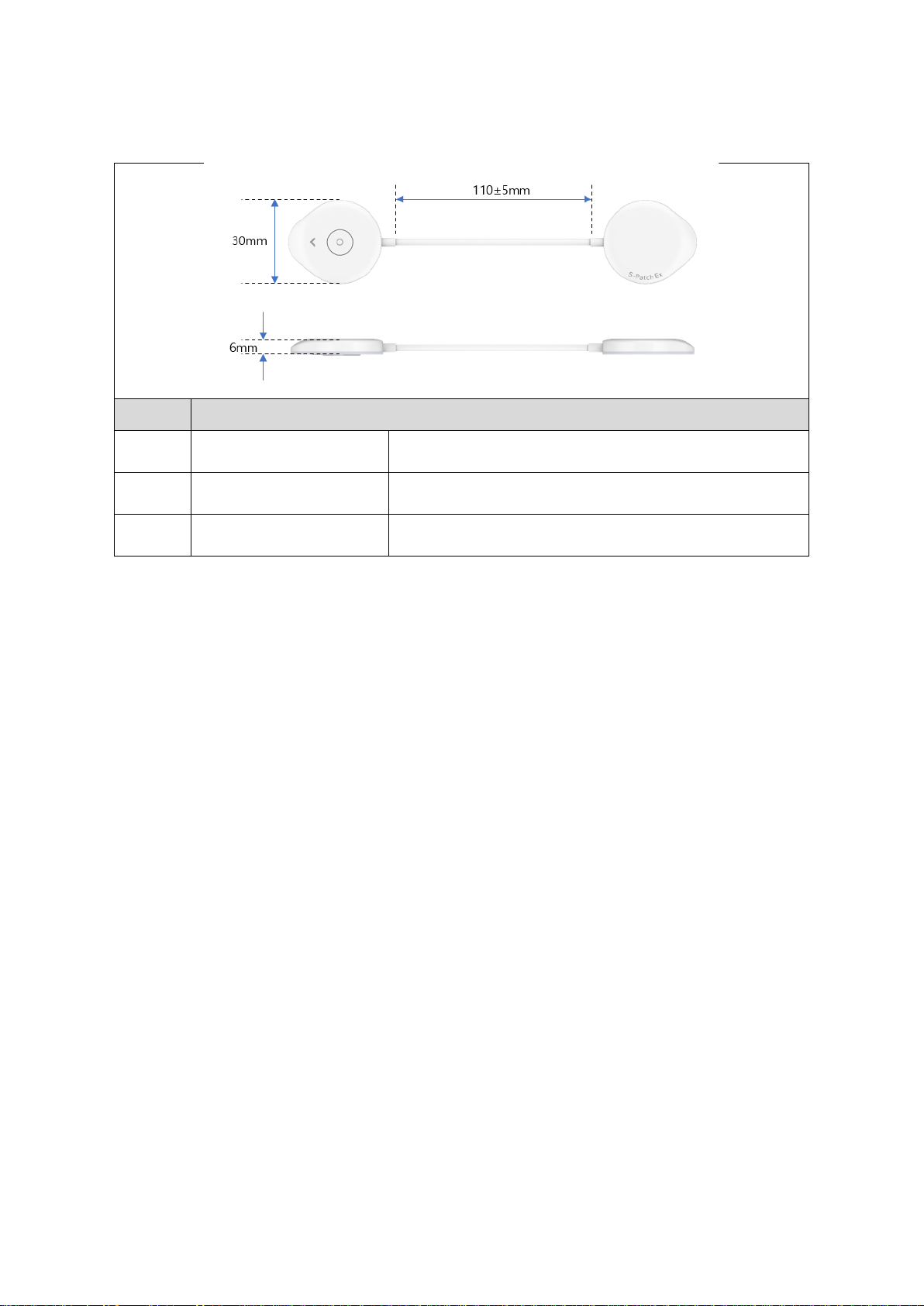
4.2 Components Dimensions............................................................................................. 10
4.3 Product Use and Storage Conditions ............................................................................ 10
4.3.1 Conditions for Usage ........................................................................................ 10
4.3.2 Conditions for Storage & Transport .................................................................... 10
5 Product Interoperability ........................................................................................................ 11
5.1 Compatible Accessories............................................................................................... 11
5.2 Compatible Software .................................................................................................. 12
5.2.1 S-Patch Ex Mobile Application............................................................................ 12
5.2.2 3rd-Party Cloud-Based ECG Viewing Platform ..................................................... 12
6 S-Patch Ex Operating Instructions (for healthcare professional) .............................................. 13
6.1 Preparation ................................................................................................................ 13
6.1.1 Inserting the battery......................................................................................... 13
6.1.2 Patient skin preparation .................................................................................... 13
6.1.3 Instruction for patient....................................................................................... 14
6.2 Turning On S-Patch Ex................................................................................................ 14
6.3 Connecting Electrodes ................................................................................................ 14
6.4 Applying S-Patch Ex to Patient Body ............................................................................ 15
6.5 Connecting to Mobile App ........................................................................................... 15
6.6 Preparing Returned S-Patch Ex for Next Patient............................................................ 16
6.7 Cleaning S-Patch Ex.................................................................................................... 17
7 S-Patch Ex Operating Instructions (for patient) ...................................................................... 18
7.1 Operate S-Patch Ex .................................................................................................... 18
7.1.1 (Optional) Logging symptoms ........................................................................... 18
7.1.2 (Optional) Removing and Reapplying S-Patch Ex ................................................ 18
7.2 Test Completion ......................................................................................................... 18
7.2.1 Auto Completion............................................................................................... 18
7.2.2 Removing S-Patch Ex and Disposal of Accessories .............................................. 18
7.3 Returning S-Patch Ex .................................................................................................. 19
8 Troubleshooting ................................................................................................................... 19
9 Specifications....................................................................................................................... 20
9.1 S-Patch Ex Specifications ............................................................................................ 20
9.2 FCC Compliance (FCC ID: 2AYDNS-PATCH) .................................................................. 21
9.3 Guidance and Manufacturer’s Declaration – Electromagnetic Emissions .......................... 22
9.4 Guidance and Manufacturer’s Declaration – Electromagnetic Immunity .......................... 23
9.5 Recommended separation distances between portable and mobile RF communications
equipment and the S-Patch Ex .............................................................................................. 25
10 Security Information ............................................................................................................ 26
10.1 System connectivity and Data flow diagram ................................................................. 26
10.1.1 System connectivity.......................................................................................... 26
10.1.2 Data flow......................................................................................................... 26
10.2 Sensitive information stored and transmitted................................................................ 28
10.2.1 Information storing .......................................................................................... 28