Finecare™ FIA Meter Plus(Model No.: FS-113)
Version: 10/03/2016 Guangzhou Wondfo Biotech Co., Ltd.
- 4 -
This manual contains the instructions for the operation of the Finecare™ FIA Meter Plus and for the
sample testing procedures. For specific tests’procedure please refer to the Instruction for Use of specific
products.
Finecare™ FIA Meter Plus manufactured by Guangzhou Wondfo Biotech Co., Ltd., is a portable
instrument for fluorescence detection to quantify concentration of various kind of analytes in human
blood or urine. The Test Device and instrument are for in vitro diagnostic use only. Finecare™ FIA
Meter Plus is intended for professional use.
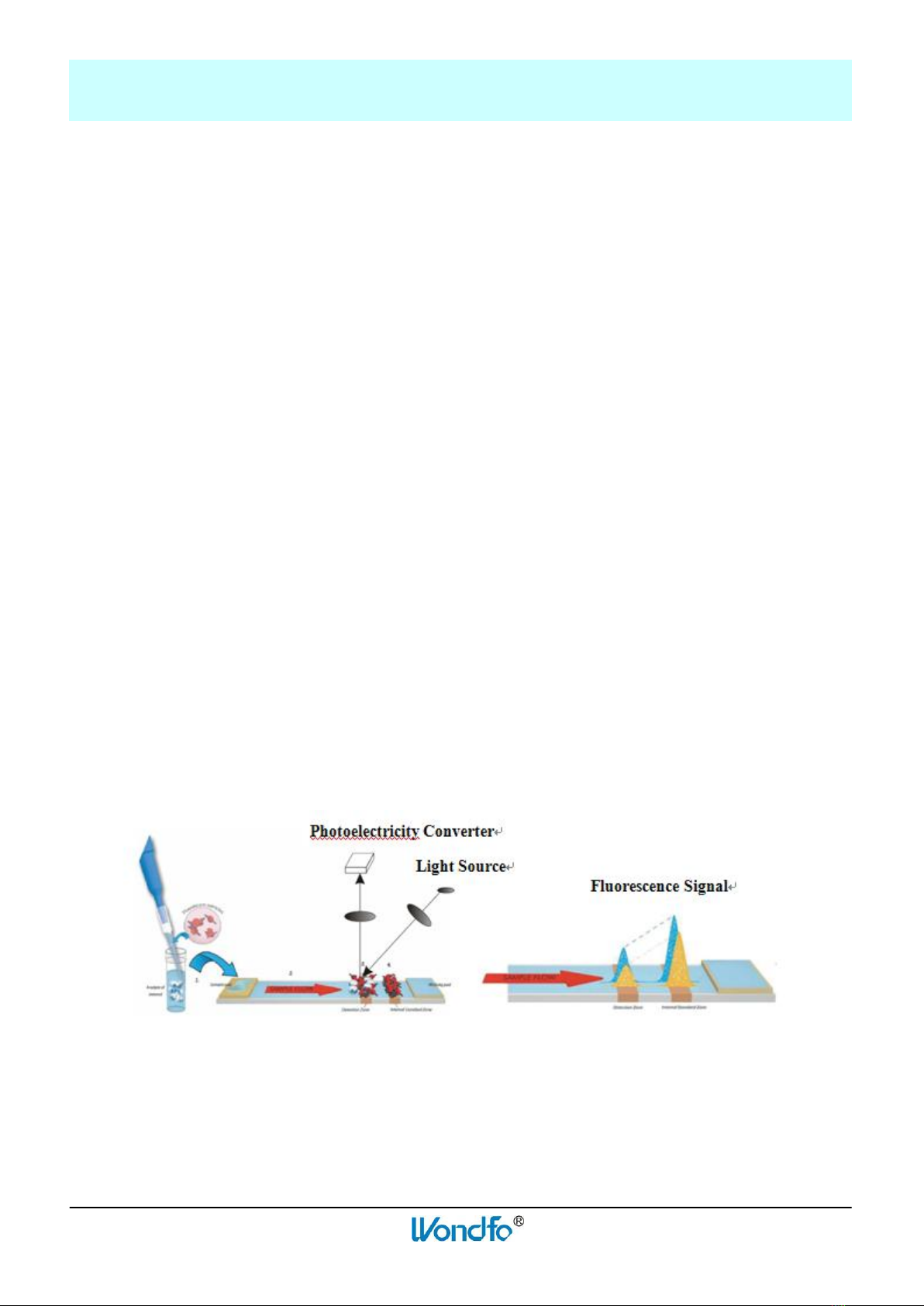
Finecare™ FIA Meter Plus uses an LED as the Excitation Light Source. The emitted light from the
fluorescence dye is collected and converted into an electrical signal. The signals are closely related to
the amount of fluoresceing dye molecules present on the spot under examination.
After a buffer-mixed sample is applied to the Test Device, the Test Device is inserted into Finecare™
FIA Meter Plus and the concentration of the analyte is calculated by a pre-programmed calibration
process. Finecare™ FIA Meter Plus can only accept Test Devices that are designed specifically for use
with this instrument.
Finecare™ FIA Meter Plus is equipped with a built-in Test Device Holder and does not require an
external holder to place the Test Device in. The Test Device Holder will appear through the opening on
the front surface of the instrument.
The Power ON/OFF Switch, located on the left of the instrument, which powers the Finecare™ FIA
Meter Plus. The instrument has a built-in printer.
Figure 1 Working Principle
Section
Ⅰ
Introduction and Principle