Contents
I Product Introduction ..................................................................................................1
I.1 Analyser Structure ...........................................................................................1
I.2 Intended Use....................................................................................................1
I.3 Technical Specifications...................................................................................1
II Contents.....................................................................................................................2
III Installation.................................................................................................................3
III.1 Installation........................................................................................................3
III.2 Instructions ......................................................................................................3
III.3 Operation Procedures......................................................................................3
III.4 Warnings..........................................................................................................4
IV Software Introduction................................................................................................5
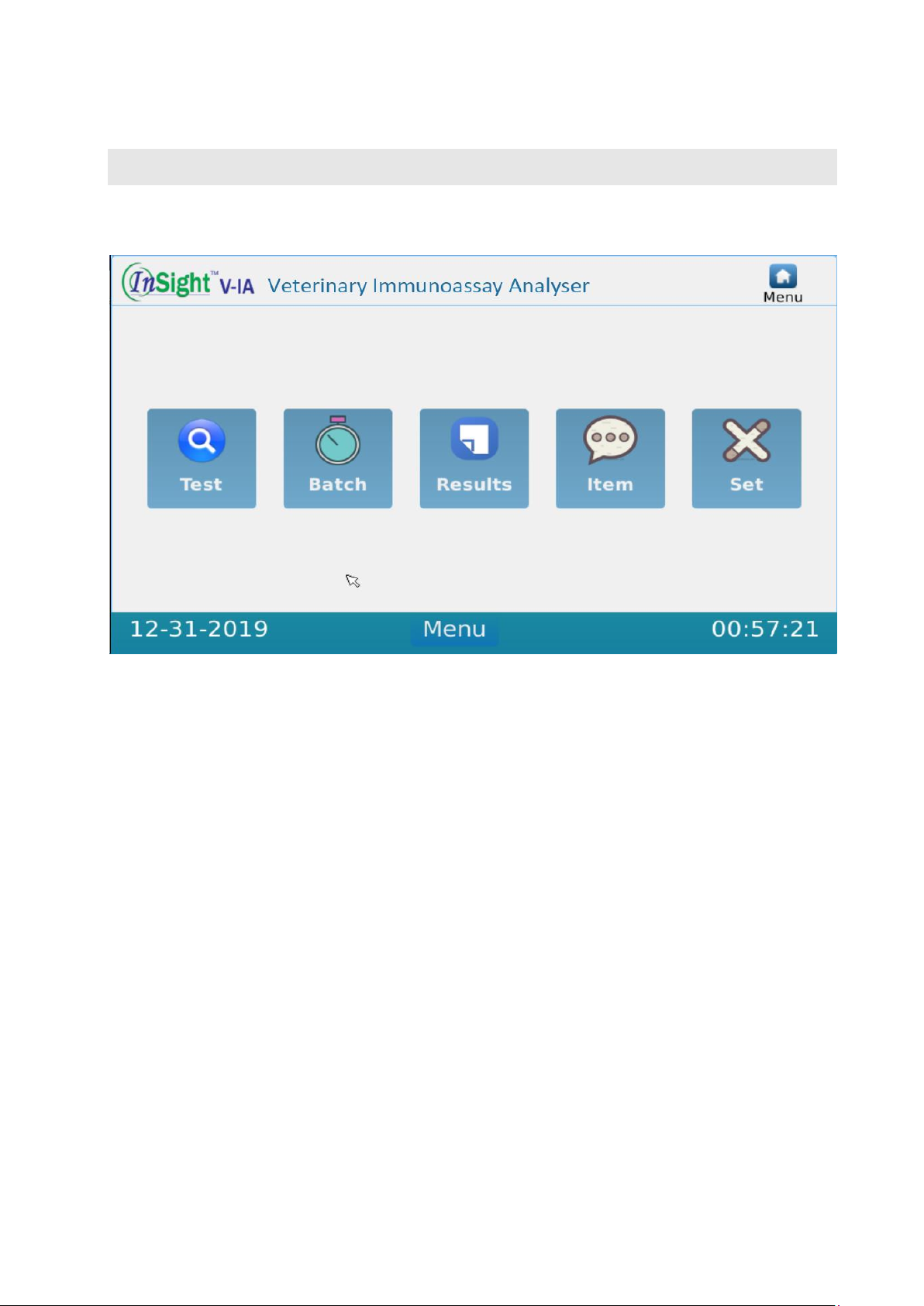
IV.1 Main Interface..................................................................................................5
IV.2 Testing Interface ..............................................................................................6
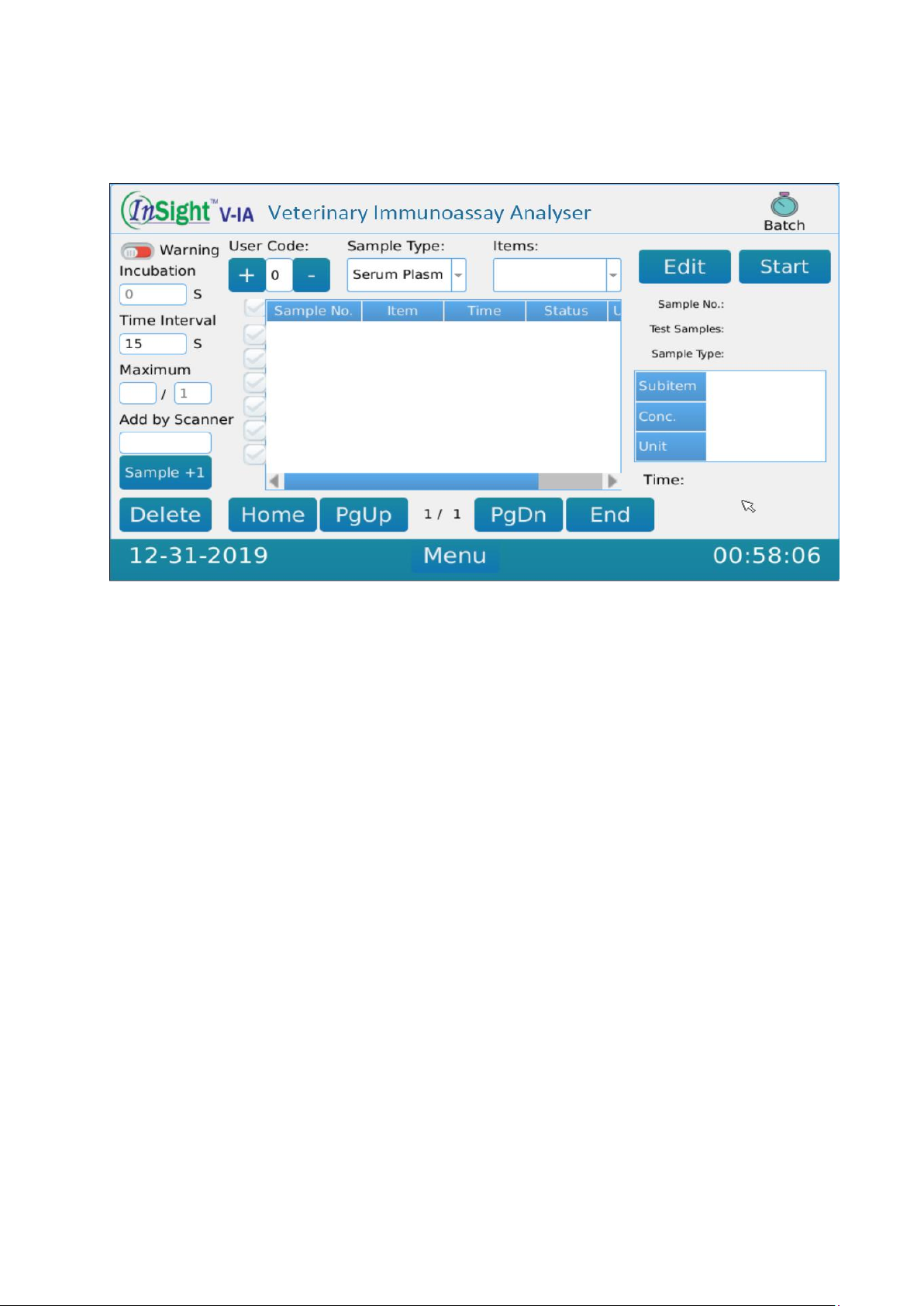
IV.3 Batch Testing ...................................................................................................7
IV.4 Results Record Interface .................................................................................8
IV.5 Item Interface...................................................................................................9
IV.6 Settings..........................................................................................................10
V Quality Control.........................................................................................................13
VI Further Product Information....................................................................................13
VI.1 Security Classification of Medical Electrical Equipment .............................13
VI.2 Contraindications.........................................................................................13
VI.3 Warnings, Precautions and Limitations........................................................13
VII Maintenance and Care ..........................................................................................18
VII.1 Daily Maintenance and Care ......................................................................18
VII.2 Troubleshooting - Common Faults and Solutions.........................................18
VII.3 Error Codes..................................................................................................19
VIII Interpretation for Medical Device Label ................................................................23
VIX Transportation Conditions ....................................................................................23
VIX.1 Transportation .............................................................................................23