Instruction Manual
K SERIES Cryostorage System
IM: CryoCeK
Revision: 02
Approved by: CB
Page 2of 87
CONTENTS
1 USER DIRECTIONS.........................................................................................................................................5
2 SAFETY PRECAUTIONS................................................................................................................................6
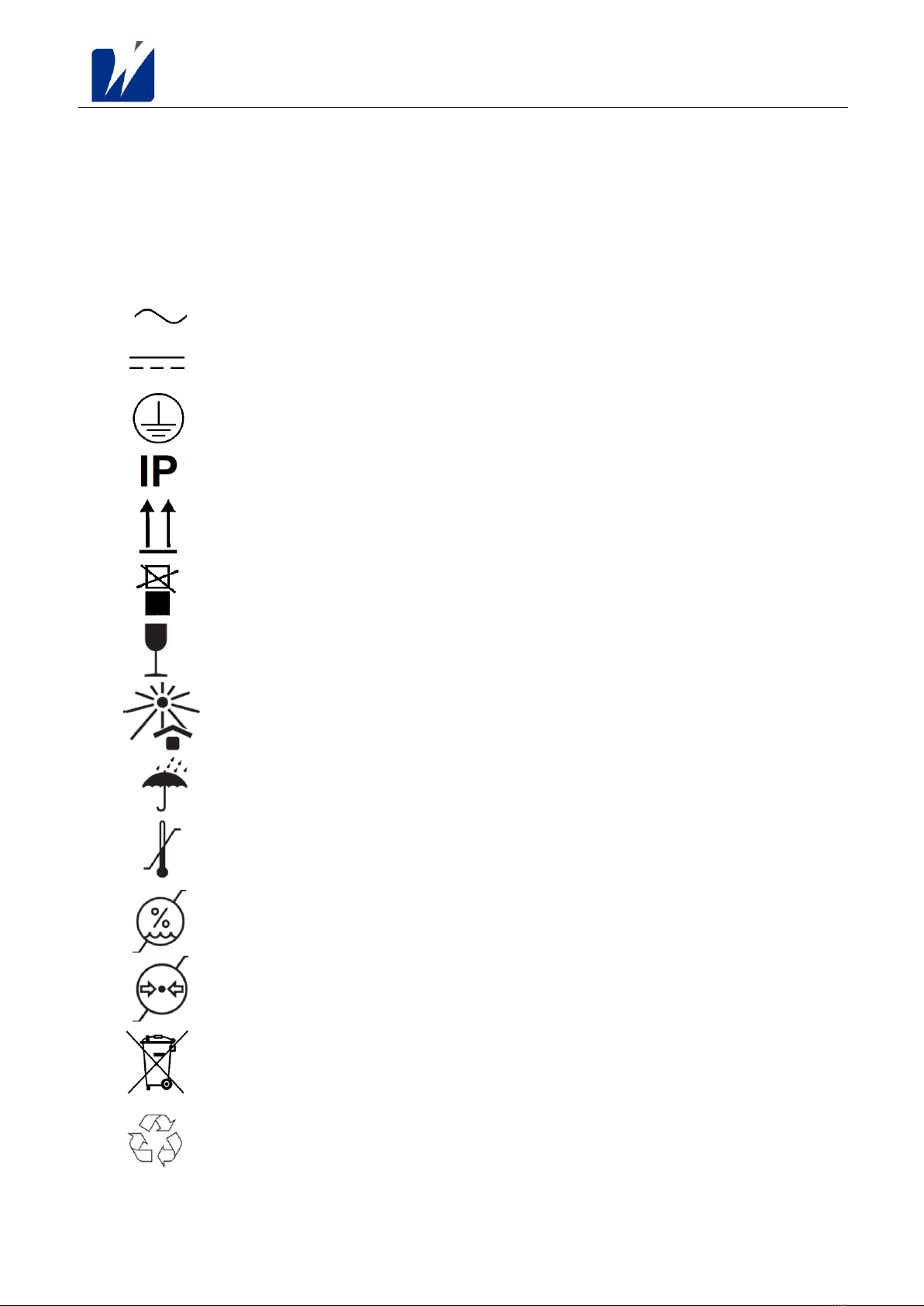
3 SYMBOLS ..........................................................................................................................................................9
4 DEVICE DESCRIPTION ................................................................................................................................11
4.1 DEVICE INTENDED USE.......................................................................................................................11
4.2 DEVICE INTENDED LOCATION AND USER.....................................................................................11
4.3 DEVICE OVERVIEW AND KEY FEATURES......................................................................................12
4.4 CRYOSTORAGE SPECIFICATIONS...................................................................................................14
4.5 INVENTORY CONTROL SYSTEM SPECIFICATIONS.....................................................................15
5 INSTALLATION OF DEVICE.........................................................................................................................20
5.1 UNPACKING AND INSPECTION..........................................................................................................20
5.2 CONDITIONS OF OPERATION AND STORAGE..............................................................................21
5.3 LIQUID NITROGEN SUPPLY CONNECTION....................................................................................22
5.4 ELECTRICAL POWER SUPPLY CONNECTION...............................................................................23
5.5 FIRMWARE...............................................................................................................................................23
6 OPERATION OF DEVICE..............................................................................................................................24
6.1 SELECTING CRYOPROTECTANTS ...................................................................................................24
6.2 INITIAL FILL..............................................................................................................................................24
6.3 NORMAL FILL CYCLE............................................................................................................................25
6.4 ADDING AN INVENTORY CONTROL SYSTEM................................................................................27
7 OPERATION OF LIQUID NITROGEN LEVEL CONTROL SYSTEM .....................................................28
7.1 CONTROLLER INITIAL START UP......................................................................................................28
7.2 CONTROLLER SET UP..........................................................................................................................29
7.3 CONTROLLER CONFIGURATION AND CALIBRATION .................................................................37
7.4 CONTROLLER OTHER SETTINGS (ENGINEER MENU)................................................................48
7.5 CONTROLLER IN NORMAL OPERATION.........................................................................................48
7.6 CONTROLLER ALARMS........................................................................................................................49
7.7 CONTROLLER RESET...........................................................................................................................52
8 CARE AND MAINTENANCE OF DEVICE..................................................................................................54
8.1 GENERAL CONSIDERATIONS............................................................................................................54
8.2 INSPECTION AND MAINTENANCE SCHEDULE..............................................................................55
8.3 CLEANING THE STRAINER..................................................................................................................56
8.4 DEFROSTING THE CRYOSTORAGE SYSTEM................................................................................57
8.5 CLEANING AND DISINFECTING THE CRYOSTORAGE SYSTEM...............................................58