chapter
4
Headline
Headline
Chapter 1 Description of Chapter
2
Intended Use
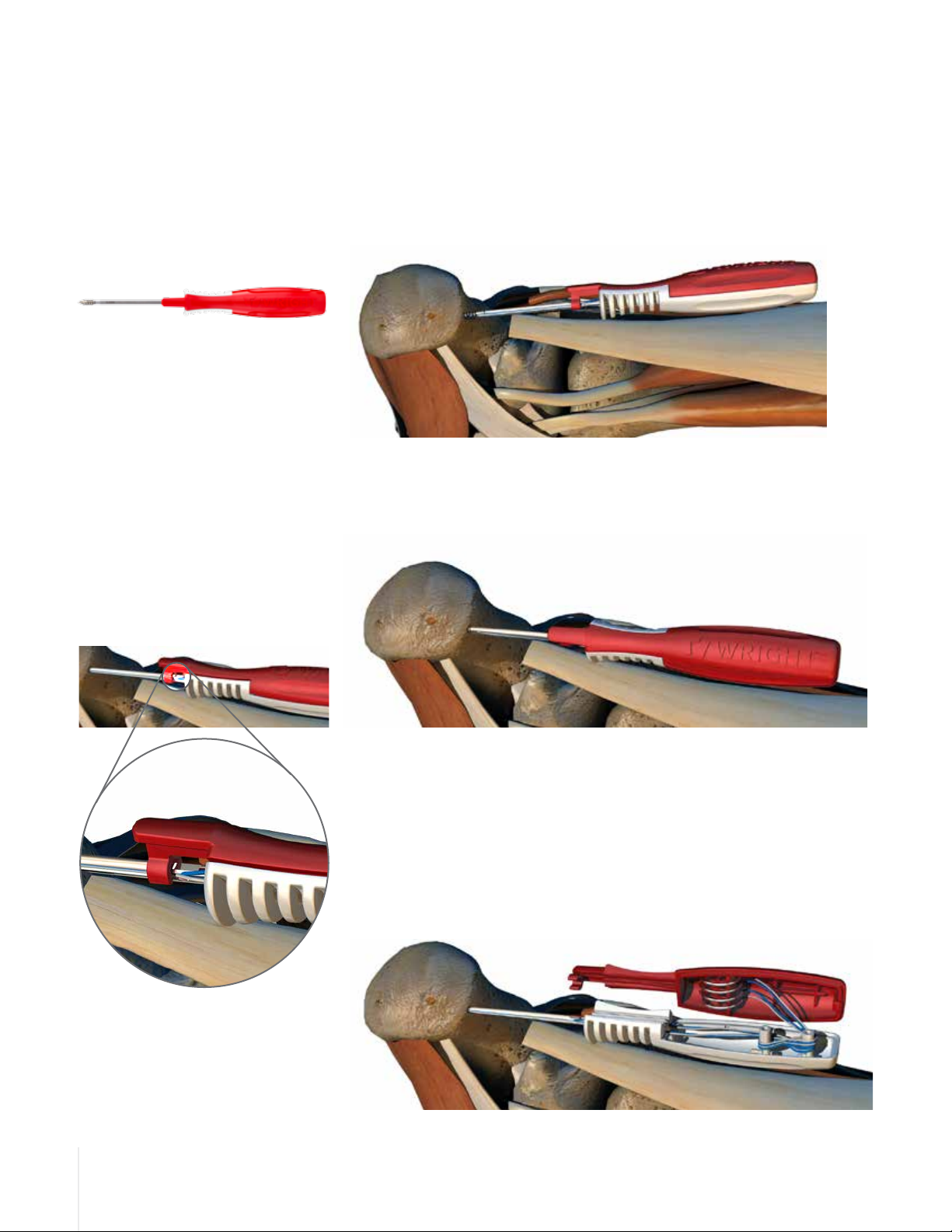
Description
The GRAVITY® Titanium Suture Anchor System features sterile, single-use
devices for use in soft tissue fixation procedures. The implants are available in a
range of sizes and feature anchors threaded with braided polyethylene sutures.
Indications
The GRAVITY® Titanium Suture Anchor System is indicated for use:
»In the repair of shoulder instability, secondary to Bankart lesion, rotator cu
tear, slap lesion, acromioclavicular separation, biceps tenodesis, deltoid tear/
separation, or capsular shift or capsulolabral reconstruction
»In the repair of elbow instability, secondary to biceps tendon detachment,
tennis elbow, or tear. Separation or tear of the ulnar collateral ligament, or
radial collateral ligament.
»In the repair of hand/wrist instability, secondary to tear or separation of the
scapholunate ligament, ulnar collateral ligament, or radial collateral ligament
»In the repair of knee instability, secondary to tear or separation of the medial
collateral ligament, lateral collateral ligament, patellar tendon, or posterior
oblique ligament, or secondary to iliotibial band tenodesis
»In the repair of foot/ankle instability, secondary to tear or separation
of the Achilles tendon, lateral stabilization tendons/ligaments, medial
stabilization tendons/ligaments, midfoot tendons/ligaments,or metatarsal
tendons/ligaments, tendon transfers, tendon reattachments, and ligament
reconstructions of the midfoot, forefoot, and hindfoot procedures associated
with flatfoot reconstruction, hindfoot deformity, midfoot reconstruction,
lateral/medial ankle reconstruction or instability, Hallux Valgus or Varus, and
MTP instability including:
»Achilles reattachment/reconstruction
»Flexor digitorum longus transfer
»Flexor hallucis longus transfer
»Extensor hallucis longus transfer
»Brostrom procedures
»Peroneal tendon relocation
»Capsule repair
»Deltoid reconstruction / reattachment
»Plantar plate repair
»Spring ligament repair
Chapter 2 Intended Use