acumed Polarus 3 User manual
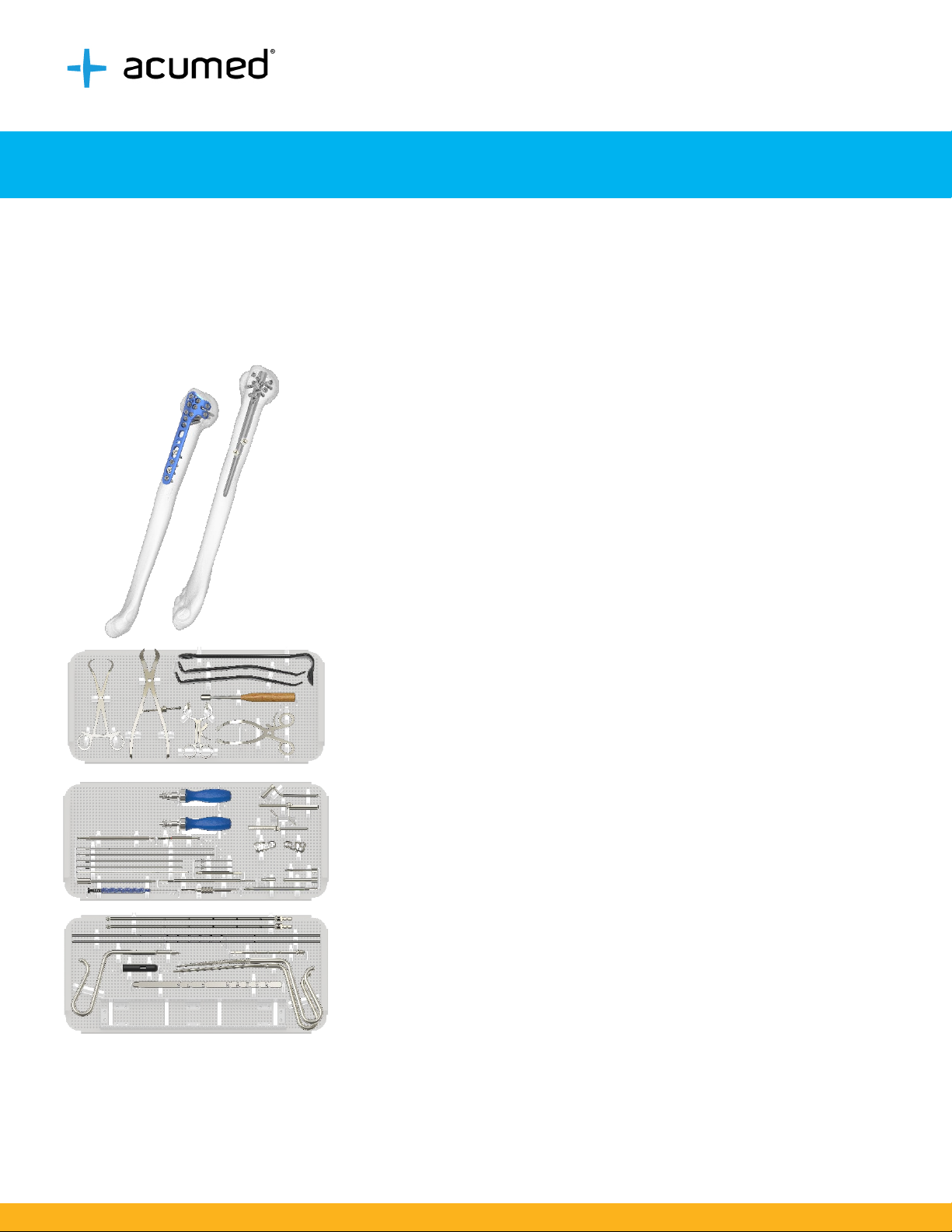
Polarus® 3 Solution Plates and Nails
US
DA
DE
EL
EN
ES
FI
FR
IT
NB
NL
PT
SV
TR
Instructions for use...................2
Brugsanvisning ......................11
Gebrauchsanweisung ............21
Οδηγίες χρήσης .....................32
Instructions for use.................43
Instrucciones de uso ..............52
Käyttöohjeet ...........................62
Mode d’emploi........................72
Istruzioni per l’uso ..................82
Bruksanvisning.......................92
Gebruiksinstructies ..............103
Instruções de utilização........112
Bruksanvisning.....................122
Kullanım talimatları ..............132

U.S. English – US / PAGE 2
US
Instructions for use
Polarus® 3 Solution Plates And Nails
These instructions are intended for the Operating Surgeon and supporting Healthcare
Professionals. The US instructions are intended for users in the United States and its
territories.
Rx only
DESCRIPTION
The Polarus® 3 Solution of bone plates, intramedullary nails, screws and accessories is designed to provide
fixation for fractures of the humerus while they heal.
INDICATIONS FOR USE
The Acumed Polarus® 3 Solution includes plates, nails, screws and accessories designed to address
fractures, fusions, and osteotomies of the humerus.
CONTRAINDICATIONS
•Active or latent infection
•Sepsis
•Insufficient quantity or quality of bone, osteoporosis
•Soft tissue or material sensitivity
•Patients who are unwilling or incapable of following post-operative care instructions
These devices are not intended for screw attachment or fixation to the posterior elements (pedicles) of the
cervical, thoracic, or lumbar spine.
WARNINGS & PRECAUTIONS
Warning:
•
For Proximal and Long Nail
: Use only 4.3 mm low-profile hexalobe screws in the proximal portion of the
nail. Do not use 3.5 mm nonlocking low-profile hexalobe screws, as there is significant risk of these
backing out. During implant removal, locate and remove the distal screws before using the Multiple
Contact Hammer. Failure to do so could result in breaking the screws, nail, or removal instrument.
•
For Proximal Humerus:
Standard and Posterior Plates:
Replacing a 3.5 mm nonlocking low-profile
hexalobe screw with a 4.3 mm low-profile hexalobe screw in the humeral head is recommended to
prevent screw migration. If dense bone is encountered when implanting 4.3 mm low-profile hexalobe
screws, a Polarus 3 4.3 mm Screw Tap (80-1623) and Polarus 3 Tap Sleeve (80-1593) are available. If
using the Posterior Plate, screws longer than 26 mm should not be used in the posterior tabs in order
to avoid screw interference. Do not bend tabs on these plates past 20 degrees; do not bend more than
once.
•
For System:
While they are provided together for convenience, a plate and a nail should not be used on
the same fracture.
•The treatment or implant may fail, including sudden failure, as a result of:
-Loose fixation and/or loosening
-
Stress, include stress from inappropriate bending of the implant during surgery
US

Instructions for use U.S. English – US / PAGE 3
US
-Stress concentrations
-Stress of weight bearing, load bearing, or excessive activity
•Failure is more likely if the implant experiences increased loads due to delayed union, nonunion, or
incomplete healing. Failure is more likely if the patient does not follow post-operative care instructions.
•Nerve or soft tissue damage may result from surgical trauma or the presence of an implant.
•Instrument breakage or damage, as well as tissue damage, may occur when an instrument is subjected
to excessive loads, excessive speeds, dense bone, improper use or unintended use.
•Implants may cause distortion and/or block the view of anatomic structures on radiographic images.
Caution:
•The implants and instruments are intended only for professional use by a licensed physician.
•Do not use or re-sterilize an implant provided in sterile packaging if the package has been damaged.
The sterility may be compromised and the cleanliness of the implant may be uncertain. Report
damaged packaging to your distributor or Acumed.
•Do not use the sterile product past the use-by date. Refer to the device label.
•Do not reuse single use surgical instruments. The instrument may suddenly fail as a result of previous
stresses.
•Do not resharpen drill bits or reamers as these devices have critical dimensions and geometries that
cannot be restored once the instrument has been consumed.
•Do not use chemical disinfection methods as chemical residues may affect steam sterilization.
•Do not block holes in the case or trays, for example with labels, as this may adversely affect steam
penetration and sterilization.
•Screws, tacks, Kirschner wires, guidewires, cutting instruments, and similar devices may be sharp.
Observe hospital procedures, practice guidelines, and/or government regulations for the proper
handling and disposal of sharps.
ADVERSE EFFECTS
Possible adverse effects include:
•Pain, discomfort, or abnormal sensations, nerve or soft tissue damage, necrosis of bone or tissue, bone
resorption, or inadequate healing from the presence of an implant or due to surgical trauma.
•Implant fracture due to excessive activity, prolonged loading upon the device, incomplete healing, or
excessive force exerted on the implant during insertion. Implant migration and/or loosening may occur.
•Metal sensitivity, histological, allergic or adverse foreign body reaction resulting from implantation of a
foreign material. Consult our document “Metal Sensitivity Statement” at www.acumed.net/ifu.
SURGICAL TECHNIQUE
Acumed offers one or more Surgical Techniques to promote the safe and effective use of this system.
Consult our Surgical Techniques at www.acumed.net.
Important: Surgical techniques may contain important safety information.
Instructions for use U.S. English – US / PAGE 4
US
Important: The instruments and implants in this system are intended to be used by suitably trained
and qualified surgeons in a hospital operating room setting. Before treatment, the surgeon is
advised to read and fully understand all instructions and communicate to the patient any relevant
medical information provided therein, including the use, limitations, risks (safety communications),
and possible adverse effects of the proposed treatment.
Consult the most recent versions of the Instructions for Use and Surgical Techniques as they are
subject to change. Contact Acumed or an authorized agent to request any additional information.
MRI SAFETY INFORMATION
The implants have not undergone testing for safety in the MR environment. Consult our publication
“Acumed Implants in the MR Environment” at www.acumed.net/ifu for more information.
LIFETIME
•Multiple-use instruments have a lifetime that is affected by usage, handling, and processing. Assess
multiple-use instruments for fitness during the pre-sterilization inspection.
•Sterile parts may be implanted up to the date of expiration indicated on the label.
STERILITY
•Implants and instruments may be provided either sterile or non-sterile as indicated on the label.
•Non-sterile devices are intended to be sterilized before use.
•Devices purchased and received sterile were exposed to a minimum dose of 25.0 kGy gamma radiation
to obtain a minimum sterility assurance level of 10-6.
IMPLANTS
MATERIALS
•The implants are made of PEEK, or titanium alloy per ASTM F136.
•Consult our document “Metal Sensitivity Statement” at www.acumed.net/ifu for the chemical
composition of Acumed metal implants.
SINGLE USE
•Implants are intended for single use only, as indicated on the label.
•Do not reuse single use implants as this may increase the risks of failure and cross-contamination.
•Dispose of any unused implant that is contaminated with human blood or tissue. Do not process a
contaminated implant.
IMPORTANT
•For safe and effective use, the surgeon must be thoroughly familiar with the implant, the methods of
application, instruments, and the recommended surgical technique.

Instructions for use U.S. English – US / PAGE 5
US
•
Physiological dimensions limit implant sizes. Select the type and size of implant that best meets the
patient’s requirements for close adaptation and firm seating with adequate support.
•Implants are not designed to withstand the stresses of full weight or load bearing, or excessive activity.
•Improper selection or improper implantation of the device may increase the possibility of loosening or
migration.
•Do not bend the implant except as indicated in the surgical technique. Repeated or excessive bending
may weaken the implant and cause failure at a later time. Refer to the surgical technique.
•Only combine implants when they are intended for that purpose.
•Protect implants against scratching and nicking to prevent stress concentrations, which can result in
failure.
•Prevent unused implants from becoming soiled.
•The color of anodized implants may change over time due to processing. This color change does not
affect the mechanical properties of the implants.
INSTRUMENTS
MATERIALS
The instruments are manufactured from various grades of titanium, stainless steel, aluminum, silicone, and
other polymers.
MULTIPLE USE and SINGLE USE
•Instruments are intended for multiple use unless identified on the label for single use only.
•Single use instruments are intended to be disposed after use on a single patient during a single
procedure.
•Do not reuse single use instruments as this may increase the risks of failure and cross-contamination.
•Multiple use instruments are only intended for use on a single patient and a single procedure before
requiring processing.
•Multiple (limited) use instruments, such as drills and reamers, have a limited lifespan. Immediately
replace any multiple use instrument if performance becomes inadequate.
•Multiple use instruments potentially contaminated with transmissible spongiform encephalopathy (TSE)
agents shall not be processed for reuse.
IMPORTANT
•Protect instruments against scratching and nicking to prevent stress concentrations, which can lead to
instrument failure.
•Near the point of use: Wipe excess contamination from instruments and prevent any soil from drying.
Instruments with substantial or dried soil are particularly difficult to reliably process. Transport
contaminated instruments for processing as soon as possible after use.
•Avoid prolonged instrument contact with iodine and saline.
•Handle and transport soiled instruments in a manner that avoids contamination of any unused implants.
Table of contents
Languages:
Other acumed Medical Equipment manuals
Popular Medical Equipment manuals by other brands

Getinge
Getinge Arjohuntleigh Nimbus 3 Professional Instructions for use

Mettler Electronics
Mettler Electronics Sonicator 730 Maintenance manual

Pressalit Care
Pressalit Care R1100 Mounting instruction

Denas MS
Denas MS DENAS-T operating manual

bort medical
bort medical ActiveColor quick guide

AccuVein
AccuVein AV400 user manual















