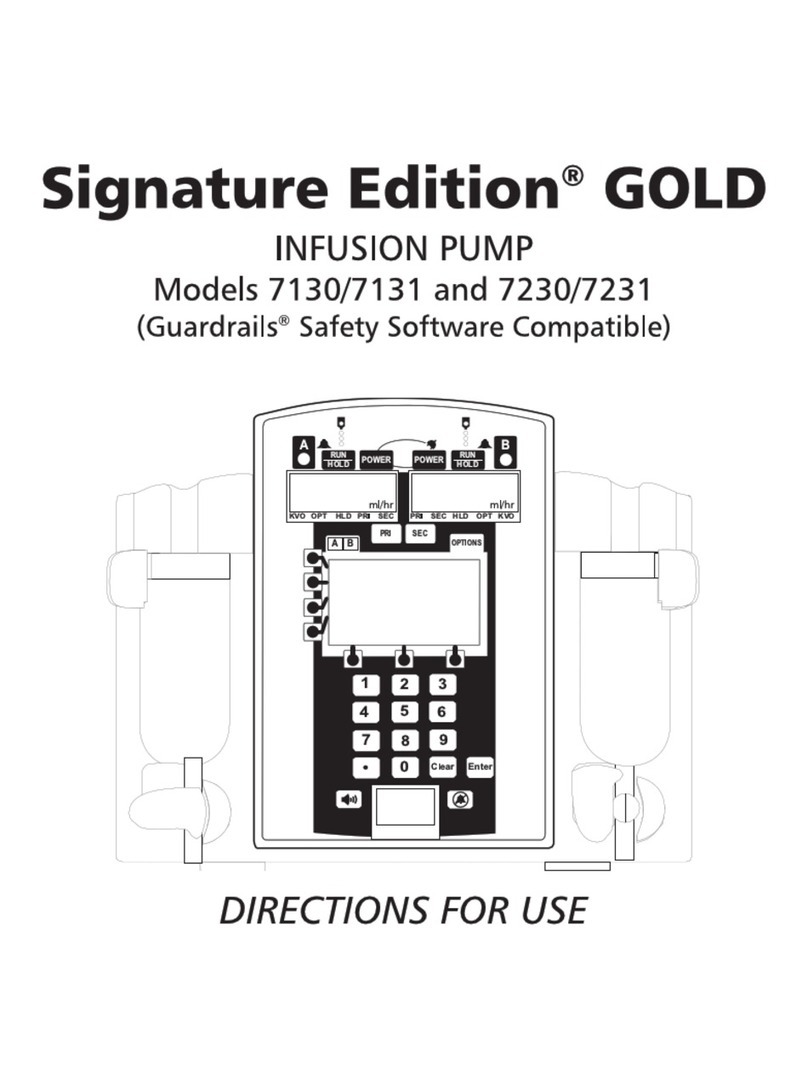
PC-2TX
LIST
OF
FIGURES
Figure
Title
Page
1-1
IMED
GEMINI
Model
PC-2TX
Volumetric
infusion
Pump/Controller
1-2
Audio
Characteristics
..............................,,..,,......,..,,................
1-6
2-4
PC-2TX
Front
and
Rear
Panel
Operating
Features
.................,..................,..
2-2
2-2
Air-In-Line
Simulator
.........................
0
페이
K
n
a
2-6
2-3
PC-2TX
Abbreviated
Test
Data
Sheet
.................................................
2-7
3-1
PC-2TX
Front
Panel
Controls
and
Indicators
.......,....,...............................
3-2
4-1
PC-2TX
Pumping
Mechanism
.......-.......
знании
ани
иен
нуна
4-2
4-2
PC-2TX
Signal
Flow
and
Interconnect
Diagram
..........................................
4-3
4-3
Cross
Section
of
Strain
Beam
Assembly
.................................
0...
4-5
4-4
AIL
Detector
Cross
Section
........................................................,..
4-9
45
Slide
Clamp
Detector
Cross
Section
...................................................
4-10
4-6
AIL/SCD
Board
Schematic
(P/N
1840-5012)
.............................................
4-11
4-7
Logic
Board
1340-5048
(Sheet
t
of
3)
.............,................................,...
4-15
4-7
Logic
Board
1320-5048
(Sheet
2
of 8)
..................................................
4-17
4-7
Logic
Board
1320-5048
(Sheet
3
of 3)
.....................,............................
4-19
4-8
Display
Board
1320-5049
(Sheet
1
of
4)
...........................,....................
4-21
4-8
DisplayBoard1320-5049(Sheet20f4)
............................................
4-23
4-8
Display
Board
1320-5049
(Sheet
3
of
4)
................................................
4-25
4-8
Display
Board
1320-5049
(Sheet
4
of
4)
....................,......................,....
4-27
4-9
Power
Supply
Board
1320-5054
(Sheet
1
of 8)
...........................................
4-29
49
PowerSuppiyBoard
1320-5054
(Sheet20f3)............................................
4-31
4-9
Power
Supply
Board
1320-5054
(Sheet
3
of
3)
........,..................................
4-33
4-10
Motor
Controller
Board
1320-5001
(Sheet
1
0Î 3)
.......,.............
eee.
4-35
4-10
Motor
Controller
Board
1320-5001
(Sheet
2
of
3)
...............,....,....................
4-37,
4-10
Motor
Controller
Board
1320-5001
(Sheet
3
of
8)
.........................................
4-39:
6-1
Parts
Identification
-
PC-2TX
Pump
Assembly
.
.......,...,...............................
6-4
6-2
Parts
Identification
-
Front
CaseAssembiy(Sheet1).
...................................
6-7
6-2
Parts
Identification
-
Front
Case
Assembly
(Sheet
2).
.....................................
6-9
6-3
Parts
Identification
-
DOOr
ASSEMblY
eneret
eve
6-12
6-4
Parts
Identification
-
Rear
Case
Assembly
{Sheet
1)
......................................,
6-15
6-4
Paris
Identification
-
Rear
Case
Assembly
(Sheet
2)
.......................................
6-17
6-5
Parts
Identification
Pole
Clamp
Assembly
..........,.............,......................
6-19
6-6
Parts
Identification
-
Power
Supply
Board
CCA
......,..............,.....................
6-26
6-7
Pertsidentification
-DisplayBoardCCA
..............................................
6-29
6-8
Parts
Identification
-
Logic
Board
CCA
e
6-35
6-9
Parts
Identification
-
Motor
Controller
CCA
..........................,......,.......,....
6-41
7-1
Universal
Test
Station
Setup
...............,..:.,,,.,.....4......
ere.
7-5
7-2
Air-in-line
Simulator.
............................
1...
lee
7-7
7-3
PC-2TX
Test
Data
Sheet
............................................................
7-8