2
Contents
1. Product overview.............................................................................................................................4
Features..............................................................................................................................................5
Key parameters...................................................................................................................................5
Applications.........................................................................................................................................6
Instrument external dimension............................................................................................................7
2. Hardware set-up guide....................................................................................................................8
Power up the instrument.....................................................................................................................8
Connect to a PC via USB....................................................................................................................8
Light indicator states...........................................................................................................................9
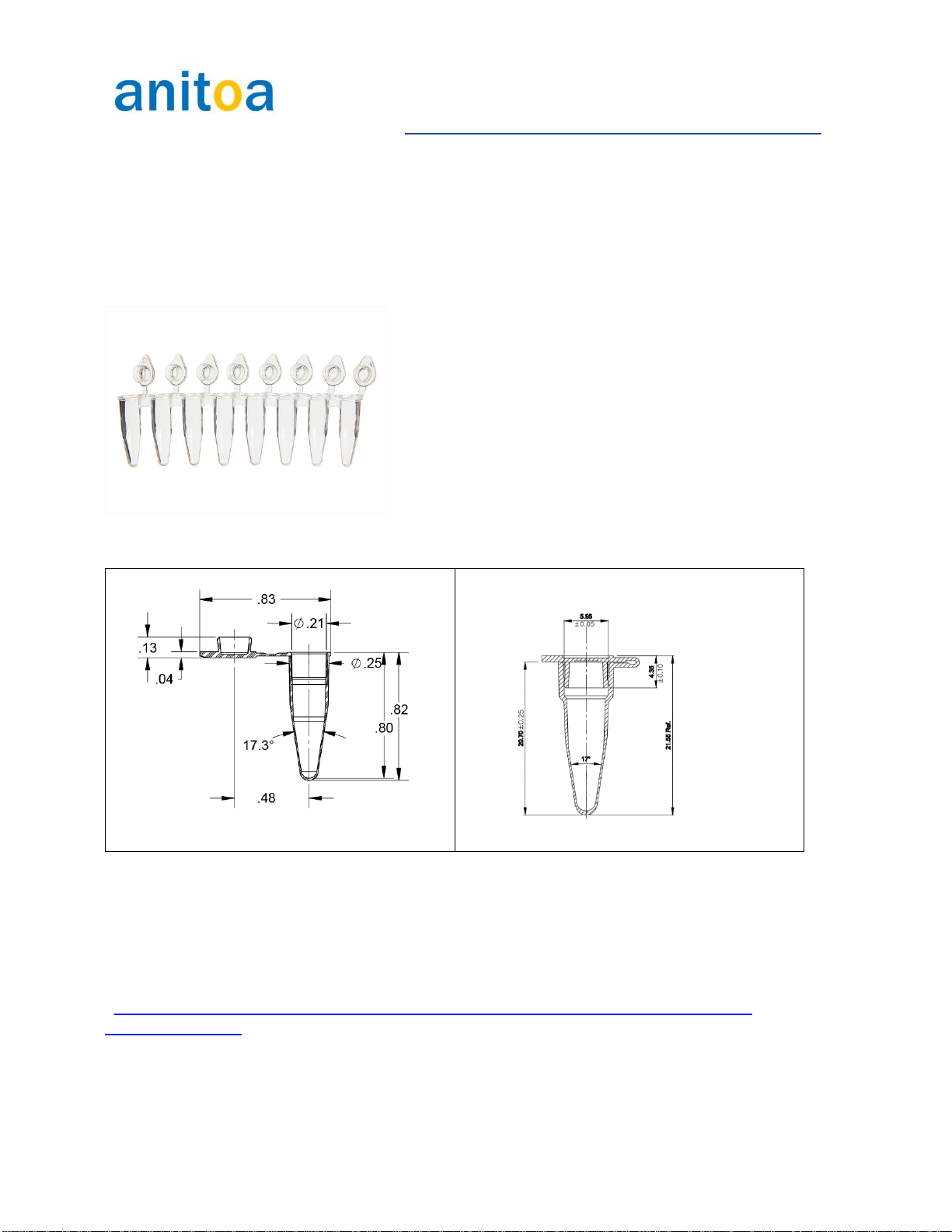
qPCR tube/plasticware requirement .................................................................................................10
Some examples of the tubes that are proven to work in Maverick instruments:...........................10
Instrument cleaning requirement ......................................................................................................12
3. Step by Step Software Guide........................................................................................................13
Install the software............................................................................................................................13
Launching the software.....................................................................................................................15
The home button ...........................................................................................................................15
Resizing the window......................................................................................................................15
The setup page –Name setup..........................................................................................................16
Samples and fluorescence channels set up..................................................................................16
Open existing experiment for template file....................................................................................17
The setup page –Cycler setup.........................................................................................................18
Melting curve analysis period........................................................................................................19
Hot lid temperature and reaction volume ......................................................................................19
Saving template.............................................................................................................................19
Auto Integration Time....................................................................................................................19
Running the experiment and monitor status .....................................................................................20
Running amplification program......................................................................................................20
Running melt analysis ...................................................................................................................21
Analysis.............................................................................................................................................22