3
Table of Symbols
Single use only. Disposable. Do not resterilize. Caution: Federal law (USA) restricts this device to sale by or on the order of a physician. Patent Pending.
DISCLAIMER OF WARRANTY AND LIMITATION OF REMEDY
THERE IS NO EXPRESS OR IMPLIED WARRANTY, INCLUDING WITHOUT LIMITATION ANY IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR
PURPOSE, ON THE APOLLO ENDOSURGERY, INC. PRODUCT(S) DESCRIBED IN THIS PUBLICATION. TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW, APOLLO
ENDOSURGERY, INC. DISCLAIMS ALL LIABILITY FOR ANY INDIRECT, SPECIAL, INCIDENTAL, OR CONSEQUENTIAL DAMAGES, REGARDLESS OF WHETHER SUCH LIABILITY
IS BASED ON CONTRACT, TORT, NEGLIGENCE, STRICT LIABILITY, PRODUCTS LIABILITY OR OTHERWISE. THE SOLE AND ENTIRE MAXIMUM LIABILITY OF APOLLO
ENDOSURGERY, INC., FOR ANY REASON, AND BUYER’S SOLE AND EXCLUSIVE REMEDY FOR ANY CAUSE WHATSOEVER, SHALL BE LIMITEDTO THE AMOUNT PAID BY THE
CUSTOMER FOR THE PARTICULAR ITEMS PURCHASED. NO PERSON HASTHE AUTHORITYTO BIND APOLLO ENDOSURGERY, INC. TO ANY REPRESENTATION OR WARRANTY
EXCEPT AS SPECIFICALLY SET FORTH HEREIN. DESCRIPTIONS OR SPECIFICATIONS IN APOLLO ENDOSURGERY, INC PRINTED MATTER, INCLUDINGTHIS PUBLICATION, ARE
MEANT SOLELYTO GENERALLY DESCRIBETHE PRODUCT ATTHETIME OF MANUFACTURE AND DO NOT CONSTITUTE ANY EXPRESS WARRANTIES OR RECOMMENDATIONS
FOR USE OF THE PRODUCT IN SPECIFIC CIRCUMSTANCES. APOLLO ENDOSURGERY, INC. EXPRESSLY DISCLAIMS ANY AND ALL LIABILITY, INCLUDING ALL LIABILITY FOR
ANY DIRECT, INDIRECT, SPECIAL, INCIDENTAL, OR CONSEQUENTIAL DAMAGES, RESULTING FROM REUSE OF THE PRODUCT.
1. Intended Use
The Apollo Endosurgery OverStitch Sx™ Endoscopic
Suture System (ESS) is intended for endoscopic
placement of suture(s) and approximation of soft
tissue.
1.1 Contraindications
Contraindications include those specific to use of
an endoscopic suturing system, and any endoscopic
procedure, which may include, but not limited to, the
following:
- This system is not for use where endoscopic
techniques are contraindicated.
- This system is not for use with malignant tissue.
1.2 Warnings
- Do not use a device where the integrity of the
sterile packaging has been compromised or if the
device appears damaged.
- Only physicians possessing sufficient skill and
experience in similar or the same techniques should
perform endoscopic procedures.
- Contact of electrosurgical components with other
components may result in injury to the patient and/
or operator as well as damage to the device and/or
endoscope.
- Verify compatibility of endoscope size, endoscopic
instruments and accessories and ensure
performance is not compromised.
- Ensure endoscope is clean, dry, and free of
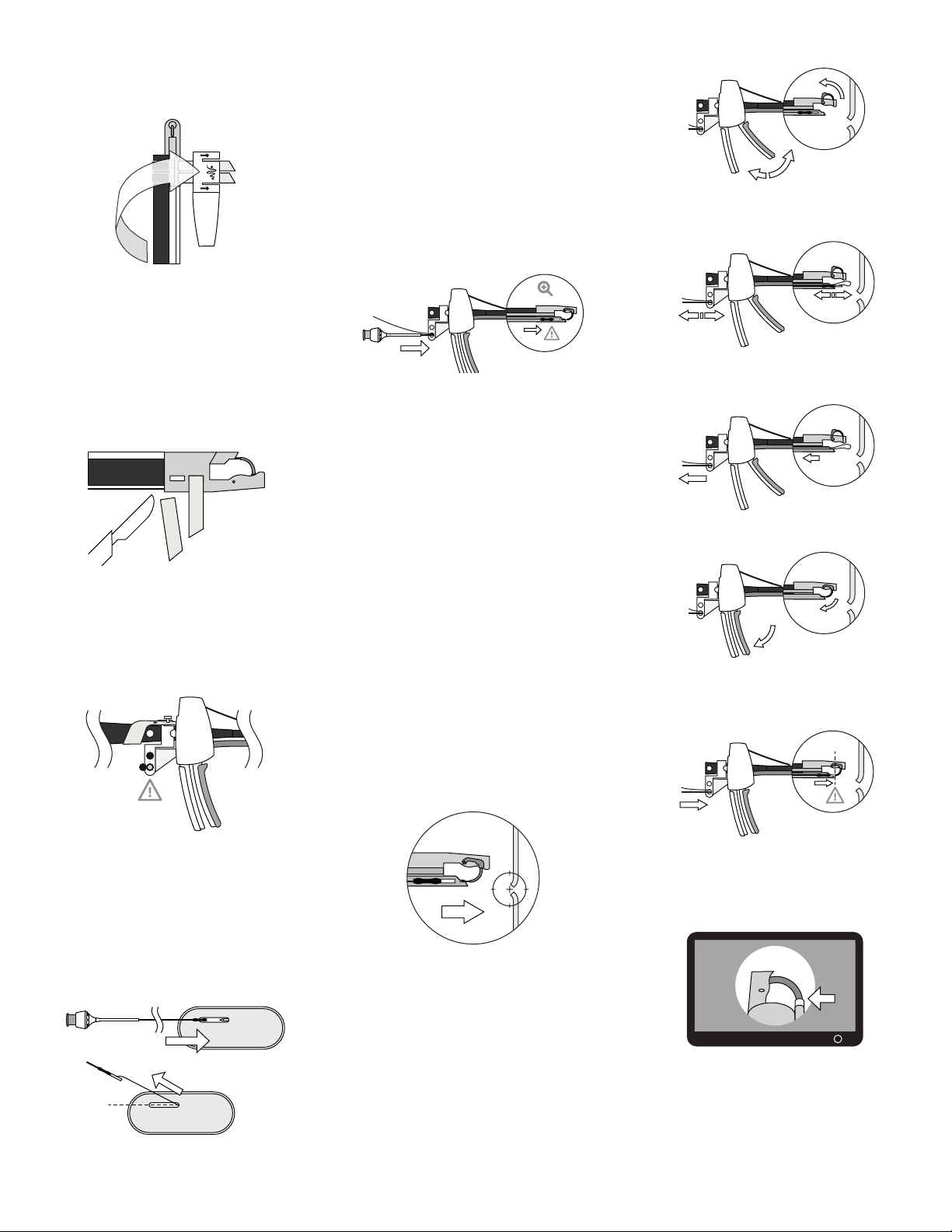
lubricants prior to device installation.
- OverStitch Sx is recommended to be used in
combination with an overtube having an inner
diameter of at least 16.7 mm
- Ensure that there is sufficient space for the Needle
to open.
- Ensure that the Handle Grip of the Endoscopic
Suturing System is closed and locked, and Actuation
Catheter slack removed, during intubation and
extubation.
- Users should be familiar with surgical procedures
and techniques involving absorbable sutures before
employing Synthetic Absorbable Sutures for wound
closure, as the risk of wound dehiscence may vary
with the site of application and the suture material
used.
- In situations where the operative site poses a risk
of harm to adjacent anatomic structures, use of
endoscopic accessories such as the OverStitch
Tissue Helix is recommended to retract the tissue
intended to be sutured away from these unseen
structures.
- If used to oversew foreign objects, such as staples,
stents, clips or mesh, it is possible for the needle
to become trapped in the foreign body, requiring
surgical intervention.
- Reuse or reprocessing of the OverStitch system
could result in device malfunction or patient
consequences to include:
-Infection or the transmission of disease
-Failure of the handle mechanism causing the
device to become locked on tissue that may
require surgical intervention
-Reduced retention on the endoscope, causing
the device to detach during use that may require
surgical intervention to retrieve
-Reduced retention of the Anchor to the Needle
Body, resulting in an inadvertent Anchor drop
causing procedural delay or requiring subsequent
intervention
-Bending of the Needle Body, preventing the
physician from driving the Needle correctly or
performing the intended procedure
-Failure of the Helix to extend fully, limiting the
ability to acquire tissue and perform intended
procedure
1.3 Precautions
- The System may only be used if purchased from
Apollo Endosurgery, Inc. or one of its authorized
agents.
- With the Endoscopic Suturing System installed, the
endoscope’s effective outer diameter is increased
by approximately 5mm.
1.4 Adverse Events
Possible complications that may result from using the
Endoscopic Suturing System include, but may not be
limited to:
- Pharyngitis / Sore throat
- Nausea and / or Vomiting
- Abdominal pain and / or Bloating
- Hemorrhage
- Hematoma
- Conversion to laparoscopic or open procedure
- Stricture
- Infection / Sepsis
- Pharyngeal, colonic and/or esophageal perforation
- Esophageal, colonic and/or pharyngeal laceration
- Intra-abdominal (hollow or solid) visceral injury
- Aspiration
- Wound dehiscence
- Acute inflammatory tissue reaction
- Death
1.5 Compatibility
- The system is compatible with endoscopes having an
insertion tube and distal diameter between 8.8mm
and 9.8mm, a working length up to 110cm, and
using overtubes having an inner diameter of at least
16.7mm.
- The system is only compatible with the sutures listed
on the first page. The system is not compatible with
other sutures on the market.
Description Symbol
Consult Instructions for Use
Do Not Re-use
Sterilized Using Ethylene Oxide
Reference Number
Description Symbol
Manufacturer
Caution: Federal law (USA) restricts this
device to sale by or on the order of a
physician
Date of manufacture
Do Not Resterilize
Description Symbol
Use By
Lot Number
Do not use if package is damaged
Authorized Representative in the
European Community